Abstract
The OLINDA/EXM version 1.0 personal computer code was created as a replacement for the widely used MIRDOSE3.1 code. This paper documents the basic function of the code and how it is similar to and different from the MIRDOSE software. Methods: After creation of the code and α- and β-testing phases, a premarket notification submission (510(k)) was filed with the Food and Drug Administration to permit marketing of the code. Permission was granted in June 2004, and the code is currently being distributed through Vanderbilt University. Not all of the technical details of the dosimetry methods have been shown here, as they have been previously documented. Results: Agreement of doses between the MIRDOSE3.1 and OLINDA/EXM codes was good, within 1%–2% in most cases. Conclusion: The extensive testing of the OLINDA/EXM code, based on comparison with literature-established dose calculations and with the widely tested and accepted MIRDOSE3.1 code, should give users confidence in its output. The OLINDA/EXM code should be easy for MIRDOSE users to adopt and for new users to understand. It will be useful in standardizing and automating internal dose calculations, assessing doses in clinical trials with radiopharmaceuticals, making theoretic calculations for existing pharmaceuticals, teaching, and other purposes.
The MIRDOSE personal computer software (1) was developed in the early 1990s and was distributed to about 2,000 users worldwide. The code automated and standardized internal dose calculations for nuclear medicine applications and found use, as well, as a teaching tool. In 2000, several factors suggested that the time had come for a rewriting of the code. First, although MIRDOSE, which was written in the BASIC programming language in the VisualBasic (Microsoft Corp.) development environment, migrated well from Windows (Microsoft Corp.) version 3.11 to version 98, migration to version 2000 was not seamless. At the same time, the U.S. Food and Drug Administration (FDA) became aware and expressed concern that the MIRDOSE code may have been used in some applications in ways that would cause it to come under the agency’s definition of a medical device. The code was subsequently withdrawn from circulation by Oak Ridge Associated Universities (2). Limited distribution was then permitted by the FDA, with careful tracking of who took possession of copies of the code. A complete rewrite in a structured language was thought desirable, as the MIRDOSE code, which had evolved over about 10 y, was written in BASIC in a nonstructured format. Therefore, the code was completely rewritten in the Java (Sun Microsystems) programming language and was renamed OLINDA (for Organ Level INternal Dose Assessment). Most of the main functions of MIRDOSE were retained, but some new models were added. In addition, a new section of code was added that allowed users to fit data to 1, 2, or 3 exponential functions; this section was called EXM (for EXponential Modeling).
Thus, OLINDA/EXM version 1.0 was created, and after α-testing (in house, testing the code against hand calculations and other known results) and β-testing (critical assessment by several outside reviewers knowledgeable about dosimetry and used to dealing with technical computer codes) were completed, a premarket notification submission (510(k)) was filed with the FDA to permit marketing of the code. Permission was granted in June 2004, and the code is currently being distributed through Vanderbilt University. This paper documents the basic function of the code and how it is similar to and different from the MIRDOSE software. Not all of the technical details of the dosimetry methods have been shown here, as they have been previously documented. The OLINDA/EXM code uses the same technical basis as the RADAR (3,4) system, which was extensively documented in 2003 (5). Readers are referred to that article for information on phantoms, organ masses, equations and relationships assumed, and other details.
MATERIALS AND METHODS
Absolutely none of the MIRDOSE BASIC coding was copied into the Java language version. All the coding was rewritten, using the structured programming approach of Java. As modules of the code were written, they were tested and then integrated into the overall program structure and tested there. Extensive testing and quality assurance were performed against hand calculations, literature-reported values of dose, and other sources for various radiopharmaceuticals (as was done with the MIRDOSE3.1 code previously). Extensive testing of OLINDA/EXM output was also performed directly against output from the MIRDOSE3.1 code, as more than 10 y of experience with this code gave high confidence in its output. Before distribution, the OLINDA/EXM code was sent to several individuals for β-testing. Their comments on code function, appearance, utility, and understandability were considered, and the code was revised accordingly. All testing results, and an extensive documentation package describing the code and its functions, were submitted to the FDA as part of its requirements for the premarket notification. Further testing and evaluation were performed by FDA representatives, and the code was modified in accordance with their suggestions before finalization.
An important conceptual approach was changed with the OLINDA/EXM code. The MIRD concept of residence time (6) has often caused confusion because of its apparent units of time (even though it really expresses the number of nuclear transitions that occur in a source region) and because of its use to represent the mean life of atoms in biologic or engineering applications. In MIRDOSE, users entered residence times for the various source organs and obtained doses per unit administered activity.
A generic equation for the absorbed dose in any target organ can be given as:
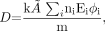 Eq. 1 where D is the absorbed dose in a target organ (rad or Gy), Ã is the cumulated activity (sum of all nuclear transitions that occurred) in a source organ (MBq-s or μCi-h), n is the number of radiations with energy E emitted per nuclear transition, E is the energy per radiation (MeV), i is the number of radiations in the decay scheme of a radionuclide, φ is the absorbed fraction (fraction of radiation energy absorbed in the target), m is the mass of the target region (g or kg), and k is the proportionality constant (Gy-kg/MBq-s-MeV or rad-g/μCi-h-MeV).
Eq. 1 where D is the absorbed dose in a target organ (rad or Gy), Ã is the cumulated activity (sum of all nuclear transitions that occurred) in a source organ (MBq-s or μCi-h), n is the number of radiations with energy E emitted per nuclear transition, E is the energy per radiation (MeV), i is the number of radiations in the decay scheme of a radionuclide, φ is the absorbed fraction (fraction of radiation energy absorbed in the target), m is the mass of the target region (g or kg), and k is the proportionality constant (Gy-kg/MBq-s-MeV or rad-g/μCi-h-MeV).
The dose equation historically used by the MIRD system and implemented in MIRDOSE3.1 was:
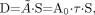 Eq. 2 where τ is the residence time (which is equal to Ã/A0, the cumulated activity divided by the patient’s administered activity [A0]), and S is given by:
Eq. 2 where τ is the residence time (which is equal to Ã/A0, the cumulated activity divided by the patient’s administered activity [A0]), and S is given by:
 Eq. 3
Eq. 3
We contend that the confusion over the idea of residence time, as defined by the MIRD Committee, can be ended by expressing the dose equation as:
 Eq. 4 where N is the number of disintegrations that occur in a source organ, and DF (the dose factor) is:
Eq. 4 where N is the number of disintegrations that occur in a source organ, and DF (the dose factor) is:
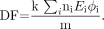 Eq. 5
Eq. 5
DF is mathematically the same as an S value as defined in the MIRD system. The number of disintegrations is the integral of a time–activity curve for a source region. The integral of this function has units of activity × time (e.g., Bq-s [1 Bq is 1 disintegration per second]). We could also use non-SI units for the number of disintegrations (e.g., μCi-hr [1 μCi-hr is equivalent to 1.33 × 108 disintegrations]). If we use the total number of disintegrations that occurs in a source region, we will get the total dose to chosen target regions. If, however, we use the number of disintegrations that occurs in a source region per unit activity administered, we define N in units of Bq-s/Bq administered (for example). We can enter the value of N, therefore, in units of time, but most users find that using that name and thinking in terms of disintegrations are inherently easier. Because OLINDA/EXM 1.0 asks for entry of values of N in Bq-h/Bq (or μCi-h/μCi), previous values of MIRD residence times are numerically equal to values of N expressed per unit activity administered.
Decay data for more than 800 radionuclides (7) were included in OLINDA/EXM, including selected α-emitters, as some (e.g., 212Bi, 213Bi, and 211At) are of current interest as nuclear medicine agents. The DF values on the RADAR site report simply the absorbed dose (mGy) per disintegration for source and target organs. OLINDA/EXM 1.0 includes the use of a radiation-weighting factor (wR), as defined by the International Commission on Radiological Protection (ICRP) (8):
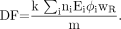 Eq. 6
Eq. 6
When radionuclides with only photon or electron emissions are involved in radiation dose calculations, whether to calculate absorbed dose or equivalent dose is not much of an issue, and the two are thought to be numerically identical, as all radiation-weighting factors to be assigned are 1.0 (8). An important issue in the calculation of dose factors for α-particles is the assignment of a radiation-weighting factor. Traditionally, a factor of 20 has been applied in radiation protection (ICRP 1979) (8). Recent radiobiologic evidence indicates that this value may be as low as 5 (9) or even 1 (10). Similar arguments apply to the use of Auger emitters (for which literature values indicate a range of potential relative biologic effectiveness values). Clearly, more investigation and guidance from regulatory and international advisory bodies is needed. OLINDA/EXM assumes a wR of 1 for photons and electrons and of 5 for α-emissions, but these values can be varied by the user. These are the default values used, but OLINDA/EXM has a form in which these values, as well as the organ masses used in the 10 whole-body phantoms, can be varied. The latter is a useful feature in situations in which an organ is known to be much larger or smaller than those in the standard phantoms. For α- and β-emissions, this results in a linear scaling of dose calculations with mass. For photons, Snyder of Oak Ridge National Laboratory showed that the photon absorbed fractions vary directly with the cube root of the mass for self-irradiation (i.e., source = target) if the photon mean pathlength is large, compared with the organ diameter (11). The photon absorbed fractions varied directly with the mass for cross-irradiation (i.e., source ≠ target). What the latter point shows is that the specific absorbed fraction for cross-irradiation does not change with differences in mass, provided the source and target are sufficiently separated and that the change in mass of one or both does not appreciably change their relative geometry. Thus, for self-irradiation, the absorbed fraction increases with the cube root of the mass of the organ, and the specific absorbed fraction decreases with the two-thirds power of the mass:
 Eq. 7
Eq. 7
The code included the 10 whole-body phantoms that were used previously in the MIRDOSE3.1 code (i.e., the 6 phantoms representing adults and children of various ages (12) and the 4 phantoms representing the adult female, either nonpregnant or at different stages of pregnancy (13)). The sphere model of MIRDOSE3.1 (used to estimate doses to tumors, animal organs, or other objects assumed to be approximately spherical and to have a uniform activity distribution) was updated with new absorbed fractions (14), but their numeric values were similar to those in the MIRDOSE code (15). These absorbed fractions permit the calculation of average dose only to spherical objects assumed to have uniform distributions of activity. Furthermore, because the spheres are isolated, only self-dose is calculated. New models, for the peritoneal cavity (16), prostate gland (17), head and brain (18), and kidneys (19), were added, and users can now enter the number of disintegrations occurring in regions of these models and obtain dose estimates. As with the MIRDOSE code, users can obtain tables of dose factors instead of dose estimates, which may be useful for teaching purposes, separate calculations, or other applications.
The EXM module allows users to fit measured kinetic data in a least-squares sense using sums of exponentials. These sums of exponentials are then integrated to determine the number of disintegrations in source regions. Users enter times of sampling, measured activity, and initial guesses for selected parameter values. Up to 10 time points and measured values can be used. One to 3 exponential terms can be selected for the modeling process, and each has 2 parameters, namely the coefficient (percentage of activity) and rate constant. The code uses nonlinear regression to converge to a local minimum in the error function (20), based on the initial starting values for each parameter, as were supplied by the user. The amount that each measured value contributes to the fitting process can be adjusted via the data weights. The regression can be performed for either decay-corrected or non–decay-corrected data.
RESULTS
Agreement of doses between the OLINDA/EXM code and other well-known and validated results was compared, usually via calculation of the ratio of the results. Code output was compared with hand calculations (for simple cases, such as β-emitters with a single emission in the decay scheme), published results (MIRD pamphlets and other well-accepted literature sources), and output from the MIRDOSE3.1 code (which had itself been thoroughly tested against hand calculations, published data, and years of extensive use by hundreds of users). Agreement was good, within 1%–2% in most cases, with 2 exceptions. In the first of these, doses to marrow and bone surfaces were somewhat different, because of modifications in absorbed fractions for these models, as documented in a recent publication (21).
In the second exception, dose to skin from skin in OLINDA/EXM from electrons was lower, because of an error in the MIRDOSE code in this calculation. This was rarely, if ever, used in nuclear medicine applications and so was not detected until the extensive OLINDA/EXM validation and verification were performed. For completeness, skin (total) is included as a source and target organ in the code, but radiopharmaceuticals rarely are taken up in significant amounts in skin. Neither MIRDOSE nor OLINDA/EXM is suitable for performing skin dose calculations from external contamination or extravasation incidents.
For recent dosimetry models (peritoneal cavity (16), prostate (17), head and brain (18), and kidney (19)), the agreement between the published S factors and the corresponding OLINDA dose factors was excellent.
An example of a portion of a dose table for a hypothetical 131I-labeled monoclonal antibody is shown in Table 1. More output is given from the program but was omitted here, including doses listed in non-SI units, calculation of effective dose and effective dose equivalent (8), and a listing of organ masses and radiation-weighting factors used in the calculations. An example of a fit to a set of hypothetical kinetic data in the EXM portion of the code is shown in Figure 1. The calculated number of disintegrations in the box labeled “Num Disintegrations” in the middle right-hand portion of the form will automatically be entered into the OLINDA kinetics input form when the form is closed.
Sample screen from EXM portion of code, fitting data for hypothetical 131I-labeled compound with 2 exponential terms. The 2 graphs show percentage of activity plotted against time.
Portion of Sample Output from OLINDA/EXM 1.0
DISCUSSION
The OLINDA/EXM 1.0 code is the first computer program to receive approval from the FDA to be distributed after 510(k) premarket notification. Its extensive testing, based on comparison with literature-established dose calculations and comparison with the widely tested and accepted MIRDOSE3.1 code, should give users confidence in its output. It will be valuable in standardizing and automating internal dose calculations, assessing doses in clinical trials with radiopharmaceuticals, making theoretic calculations for existing pharmaceuticals, teaching, and other purposes. The OLINDA/EXM 1.0 code will have a 3-y lifespan. After June 2007, OLINDA/EXM version 2.0 will probably be released, assuming that there is still a need for the code in the user community, and all indications are that there will be. If any minor bug fixes or enhancements to the code are thought necessary in the 3 y between 2004 and 2007, incremental updates (e.g., 1.1, 1.2) may be released.
The OLINDA/EXM code should be easy for MIRDOSE users to adopt. A feature in OLINDA/EXM allows users to import old MIRDOSE3.1 stp files, which contain case studies saved previously. OLINDA/EXM cannot import 100% of the information in these files, but the numbers of disintegrations, which are the most tedious to retype, are imported. OLINDA/EXM then saves its own version of these files, called cas files. The OLINDA/EXM output no longer shows the first and second most important source organs contributing to the total dose to a target organ (this information is still available on another form that can be requested) but now shows the contributions from α-, electron, and photon emissions. This feature permits users to understand how important each type of contribution was and may be useful in guiding choices of radiation-weighting factor, which is a variable that users may change. Users will probably also find useful the ability to change organ masses in the phantoms. This ability is a small step in the direction of more patient-specific dose calculations and has been done after the fact from MIRDOSE3.1 output, when it may be known that an organ is significantly larger or smaller than that assumed in the phantom. No new Monte Carlo calculations are done by OLINDA/EXM; only the simple manipulation noted in Equation 7 is performed. Another useful feature, added at the request of a β-tester of the code, is the ability to multiply the doses per unit administered activity by the actual activity given in a study and obtain total absorbed doses, in mGy and rad. All code output may be copied from the screen, printed, or sent to a text format data file for further manipulation or presentation.
OLINDA/EXM provides the user community with continuity with MIRDOSE3 in the implementation of important standardized phantom series used in internal dosimetry. The phantom series of Cristy and Eckerman (12) and of Stabin et al. (13) have been widely used and endorsed, including use by the ICRP in its radiation dose compendia for radiopharmaceuticals (22,23). A major goal in the development of the MIRDOSE codes was to standardize how doses were calculated in the user community. Variability in calculational approaches (particularly in implementation of the remainder-of-body correction (24)), models chosen, and other factors caused dose estimates published in the literature or submitted to government agencies to differ in ways that were often difficult to understand. If a standardized computer code were used by different investigators, the variability in the code output would be limited to variability in provided input and the choice of models and model assumptions. Some difficulty has continued in the evolution of bone and marrow models. Although several refinements to these models have occurred in the last 20 y, the doses provided in the OLINDA/EXM code are, for the most part, quite consistent with those in the MIRDOSE3.1 code and in the MIRD 11 S values of 1973 (21,25), thus providing continuity and confidence in the reported results (within the ability of any standardized model to predict marrow dose).
Caution must always be exercised when computer codes are used in technical computations, particularly those involving human subjects. First, the technical basis of the code, as well as the vision of the authors in how the code should be used and applied, must be thoroughly understood. Poor input will produce poor output in all cases. Misunderstanding of the proper use of the code features or misinterpretation of its output may lead to misapplication of reported results. The standardized models used in the OLINDA/EXM code represent average, healthy individuals. As with MIRDOSE (and even hand calculations done with MIRD 11 S values), the organ doses represent the average deposition of energy across the whole organ, assuming a uniform distribution of activity within that organ. The code does not provide dose distributions from nonuniform distributions of activity, dose gradients near tissue interfaces, or tumor doses (except for the ability to calculate the self-dose from isolated tumors, using the sphere models). Application of any calculated results to clinical situations must be done with the utmost of care and an appreciation for the variability of human subjects from the averages represented in the model, in particular if disease or other conditions may cause significant deviations from model conditions. The OLINDA/EXM code contains numerous notifications to the user of these issues.
Footnotes
Received Dec. 30, 2004; revision accepted Jan. 24, 2005.
For correspondence or reprints contact: Michael G. Stabin, PhD, Department of Radiology and Radiological Sciences, Vanderbilt University, 1161 21st Ave. S., Nashville, TN 37232-2675.
E-mail: michael.g.stabin{at}vanderbilt.edu








