Visual Abstract
Abstract
Fibroblast activation protein (FAP), expressed in the tumor microenvironment of a variety of cancers, has become a target of novel PET tracers. The purpose of this report is to evaluate the imaging characteristics of 68Ga-FAP-2286, present the first—to our knowledge—dosimetry analysis to date, and compare the agent with 18F-FDG and FAPI compounds. Methods: Patients were administered 219 ± 43 MBq of 68Ga-FAP-2286 and scanned after 60 min. Uptake was measured in up to 5 lesions per patient and within the kidneys, spleen, liver, and mediastinum (blood pool). Absorbed doses were evaluated using MIM Encore and OLINDA/EXM version 1.1 using the International Commission on Radiological Protection publication 103 tissue weighting factor. Results: Forty-six patients were imaged with 68Ga-FAP-2286 PET. The highest average uptake was seen in sarcoma, cholangiocarcinoma, and colon cancer. The lowest uptake was found in lung cancer and testicular cancer. The average SUVmax was significantly higher on 68Ga-FAP-2286 PET than on 18F-FDG PET in cholangiocarcinoma (18.2 ± 6.4 vs. 9.1 ± 5.0, P = 0.007), breast cancer (11.1 ± 6.8 vs. 4.1 ± 2.2, P < 0.001), colon cancer (13.8 ± 2.2 vs. 7.6 ± 1.7, P = 0.001), hepatocellular carcinoma (9.3 ± 3.5 vs. 4.7 ± 1.3, P = 0.01), head and neck cancer (11.3 ± 3.5 vs. 7.6 ± 5.5, P = 0.04), and pancreatic adenocarcinoma (7.4 ± 1.8 vs. 3.7 ± 1.0, P = 0.01). The total-body effective dose was estimated at 1.16E−02 mSv/MBq, with the greatest absorbed organ dose in the urinary bladder wall (9.98E−02 mGy/MBq). Conclusion: 68Ga-FAP-2286 biodistribution, dosimetry, and tumor uptake were similar to those of previously reported FAPI compounds. Additionally,68Ga-FAP-2286 PET had consistently higher uptake than 18F-FDG PET. These results are especially promising in the setting of small-volume disease and differentiating tumor from inflammatory uptake.
Fibroblast activation protein (FAP)–targeting radioligands have emerged as promising agents in diagnostic cancer imaging and therapy. FAP is a transmembrane glycoprotein with a limited presence in healthy adult tissue but significant expression in cancer-associated fibroblasts, which are present in the stroma of most epithelial tumors and are known to be involved in disease growth and progression (1,2). The tumorigenic nature of cancer-associated fibroblasts, and the fact that they are absent from most healthy tissue, make FAP an attractive target in the development of pantumor radiopharmaceuticals.
Most of the literature on FAP-targeting PET focuses on the FAP inhibitors (FAPIs). FAPI-04, for example, has shown excellent uptake in breast cancer, esophageal cancer, lung cancer, sarcoma, and cholangiocarcinoma, as well as superior diagnostic efficacy in primary and metastatic lesions compared with 18F-FDG across several tumor types (3,4). There is also considerable interest and promising results in abdominal malignancies and using FAPI tracers to identify peritoneal spread, which is poorly imaged with 18F-FDG (5).
FAP-2286 differs from FAPI molecules using a cyclic peptide as a binding motif rather than a quinolone-based structure; however, previous evaluations have shown similar imaging characteristics to FAPI compounds (6). For example, Pang et al. recently presented results indicating the superiority of FAP-2286 over 18F-FDG in hepatic, gastric, and pancreatic cancers (7). Preclinical and first-in-humans studies of 68Ga-FAP-2286 and 177Lu-FAP-2286 have also demonstrated prolonged tumor retention, making it a potential therapeutic target (6,8).
Here, we present our initial prospective experience with 68Ga-FAP-2286, including comparison to 18F-FDG PET when available, biodistribution in major organs, and radiation dosimetry analysis.
MATERIALS AND METHODS
Study Design and Patients
This single-arm, prospective study was performed at the University of California San Francisco with the approval of the local institutional review board (NCT04621435) and according to the ethical principles of the 1964 Declaration of Helsinki. Patients were eligible if they had histopathologically confirmed solid tumors. They were enrolled into cohort 1 (dosimetry cohort, which included patients with and without RECIST-measurable disease), cohort 2 (metastatic disease present on the basis of RECIST), or cohort 3 (no evidence of metastatic disease but at high risk for the presence of metastatic disease). All patients gave written informed consent. Data were collected in a central REDCap database. 18F-FDG PET scans were included for comparison if they were performed within 3 mo of the 68Ga-FAP-2286 PET. Imaging results in patients with bladder cancer will be reported separately.
68Ga-FAP-2286 Radiosynthesis
Radiosynthesis was conducted in an iQS 68Ga fluidic labeling module and cassette (ITM Pharma Solutions GmbH). The precursor, FAP-2286 (Clovis Oncology, Inc.), was prepared into 41-μg/50-μL ultra-high-purity water aliquots and stored at −20°C until use. Before radiosynthesis, the precursor aliquot was mixed with 800 μL of 1 M ammonium acetate and 200 μL of 0.125 M sodium ascorbate. The reactor was preheated to 120°C, and the prepared precursor was added. 68Ga was eluted from a 68Ge/68Ga generator (Eckert and Ziegler) with 5 mL of 0.1 M HCl into the reactor, yielding 68GaCl3 with radioactivity in the range of 555–1,110 MBq (15–30 mCi). The radiolabeling proceeded for 10 min, and the reaction mixture was passed through a sterilizing filter into a sterile, pyrogen-free product vial to yield 68Ga-FAP-2286. The radiolabeling yield was 95%, and radiochemical purity was 97% (n = 15).
68Ga-FAP-2286 PET Imaging
Patients were injected with 219 ± 43 MBq (allowed range, 111–296 MBq) and imaged at a mean of 61 ± 7.4 min after injection with a target uptake time of 60 min (allowed range, 50–100 min). A subset of 10 patients was also imaged at 30 and 120 min for dosimetry. Patients were imaged on either a Vision PET/CT (n = 34, Siemens Healthineers) or a 3.0-T Signa PET/MRI (n = 12, GE Healthcare). For PET/CT, continuous bed motion was used with a bed speed of 0.7 cm/s, resulting in a scan duration of 11 min and 46 s for a coverage of 495.5 cm. Noncontrast low-dose CT was used for attenuation correction, and images were reconstructed using ultraHD•PET (Siemens) time of flight with 8 iterations and 5 subsets. For PET/MRI, a whole-body PET acquisition from the mid thighs to the vertex was obtained for 3 min at each of the 6 bed positions. The following MRI sequences were obtained at each bed position: axial MR attenuation correction and axial T1-weighted images (LAVA Flex; GE Healthcare).
Image Interpretation
A board-certified nuclear medicine physician evaluated the reconstructed PET, CT, or MR images. A positive lesion on PET was defined as a focus of activity with an SUV at least 1.5 times higher than the SUVmean of the mediastinal blood pool and not attributable to physiologic distribution such as urinary excretion. A volume of interest was semiautomatically placed around each lesion, and the calculated SUVmax was recorded, including lesions that were detected on standard scans but were not positive on PET. SUVmax data were then averaged across all lesions within a given patient. To avoid clustering effects, analysis was limited to the 5 largest lesions in each individual patient. Additionally, volumes of interest were placed over the mediastinum, liver, spleen, and kidneys to measure physiologic distribution; SUVpeak was used for quantification in the kidneys. Quantitative uptake was performed at the 60-min time point for all patients.
Radiation Dosimetry
The 10 patients in the dosimetry cohort were imaged at 30-, 60-, and 120-min time points. The data from these patients were used to estimate the internal radiation dose. MIM Encore (MIM Software) was used to segment the source organs. All activity within each organ was included. OLINDA version 2.0 was used to derive values, with curve fitting performed on the EXM module of the OLINDA version 1.1 software package (9). The reference adult model with International Commission on Radiological Protection publication 103 tissue weighting factor was used (10).
Safety
The first 35 patients enrolled in the study underwent pre- and postimaging laboratory evaluation, electrocardiography, physical examination, and vital sign assessment. Subsequent patients underwent physical examination and vital sign assessment only. All patients were monitored for adverse events up to 2 h after injection and were contacted again 1–3 d later to evaluate for delayed adverse events.
Statistical Analysis
Descriptive statistics were used to describe uptake across tumor types. The Student t test was used for comparison of SUVs between 68Ga-FAP-2286 and 18F-FDG PET. P values of less than 0.05 were considered statistically significant.
RESULTS
Patient Characteristics and Safety
Forty-six patients were enrolled between January 2021 and April 2023. Patient characteristics are available in Table 1. No clinically significant changes were seen in laboratory assessments or electrocardiograms in the first 35 patients who received 68Ga-FAP-2286. Across all patients, there were no clinically significant changes in physical examination findings or vital signs after administration. No serious adverse events were reported.
Patient Characteristics
Biodistribution
SUVmean was measured in the liver (2.0 ± 0.8), spleen (0.95 ± 0.31), and blood pool (1.4 ± 0.4), and SUVpeak was measured in the kidneys (4.2 ± 1.4), at the 60-min time point in all 46 patients (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org).
Tumor Uptake and Effect of Lesion Size
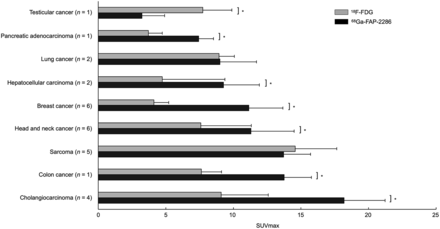
Forty-three patients had 68Ga-FAP-2286–positive lesions, including all patients in cohort 1 and nearly all patients in cohort 2. Sarcoma (SUVmax, 16.8 ± 7.5) and cholangiocarcinoma (SUVmax, 16.6 ± 6.5) demonstrated the highest uptake across tumor types (Fig. 1). The total number of lesions evaluated was 147, with a distribution by lesion size as follows: 5 lesions measured 0–5 mm; 23 lesions, 5–10 mm; 33 lesions, 10–15 mm; 17 lesions, 15–20 mm; and 69 lesions, more than 20 mm. Median SUVmax generally increased with lesion size (Supplemental Fig. 2, P = 0.037 for the 0- to 5-mm and >20-mm groups).
Tumor uptake by tumor type. SUVmax-avg represents average SUVmax of hottest lesion per patient. Error bars represent 95% CI. Average blood pool uptake (BP) was 1.4.
Comparison to 18F-FDG
Comparing 68Ga-FAP-2286 PET with 18F-FDG PET across all disease types, there was higher uptake on 68Ga-FAP-2286 PET than on 18F-FDG PET (11.5 ± 6.4 vs. 7.8 ± 5.9, P < 0.001; Fig. 2). The disease types with the highest relative uptake on 68Ga-FAP-2286 PET compared with 18F-FDG were cholangiocarcinoma (n = 4, 18.2 ± 6.4 vs. 9.1 ± 5.0, P = 0.007), breast cancer (n = 6, 11.1 ± 6.8 vs. 4.1 ± 2.2, P < 0.001), colon cancer (n = 1, 13.8 ± 2.2 vs. 7.6 ± 1.7, P = 0.001), HCC (n = 2, 9.3 ± 3.5 vs. 4.7 ± 1.3, P = 0.01), head and neck cancer (n = 6, 11.3 ± 3.5 vs. 7.6 ± 5.5, P = 0.04), and pancreatic adenocarcinoma (n = 1, 7.4 ± 1.8 vs. 3.7 ± 1.0, P = 0.01).
Comparison of paired SUVmax across various cancer types imaged with 68Ga-FAP-2286 PET vs. 18F-FDG PET. Error bars represent 95% CI. *P < 0.05.
Dosimetry
The dosimetry is reported in Table 2 (full table available as Supplemental Table 1). The highest absorbed dose for 68Ga-FAP-2286 was in the urinary bladder wall (9.98E−02 mGy/MBq), followed by the kidneys (4.31E−02 mGy/MBq), liver (2.23E−02 mGy/MBq), and spleen (7.9E−03 mGy/MBq). The effective dose was estimated at 1.16E−02 mSv/MBq.
Selected Organ-Absorbed Dose and Effective Dose for 68Ga-FAP-2286 and Other Tracers
Clinical Applications of Note
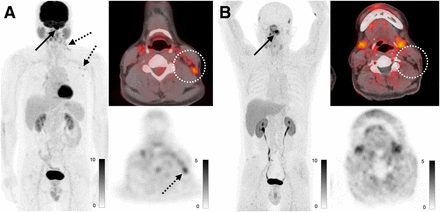
A total of 8 patients with head and neck cancer were enrolled, 2 of whom had no evidence of disease. SUVmax in primary lesions was 13.1 ± 5.5 and uptake in metastases was 11.5 ± 3.7, and there was higher uptake in metastases with 68Ga-FAP-2286 than with 18F-FDG (11.5 vs. 5.6, P < 0.001). One benefit of 68Ga-FAP-2286 compared with 18F-FDG was relatively low background uptake in mucosal, salivary, and inflammatory regions of uptake. In one case, a patient at staging had false-positive nodes on 18F-FDG PET due to a recent coronavirus disease 2019 vaccination; these nodes demonstrated no uptake on the 68Ga-FAP-2286 PET (Fig. 3).
A 58-y-old man with newly diagnosed nasopharyngeal carcinoma. (A) Maximum-intensity projections, axial fused PET, and axial PET images from 18F-FDG PET/CT demonstrate known nasopharyngeal mass with SUVmax of 23.7 (solid arrow), along with small but mildly hypermetabolic left cervical and axillary lymph nodes with SUVmax of up to 3.7 (dashed arrows, dotted circle). (B) Maximum-intensity projections, axial fused PET, and axial PET images from 68Ga-FAP-2286 PET/CT demonstrate only nasopharyngeal mass with SUVmax of 12.7 (arrow), with no uptake in the cervical lymph nodes (dotted circle). Fine-needle aspiration of left cervical node revealed reactive changes, which was attributed to recent coronavirus disease 2019 vaccine.
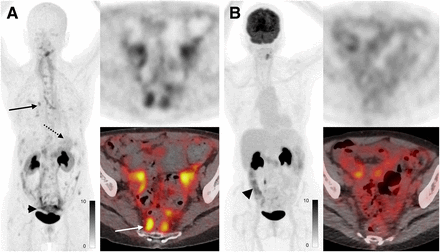
In total, 9 patients with breast cancer were enrolled. Lesion SUVmax was higher on 68Ga-FAP-2286 PET than on 18F-FDG PET in all patients (11.1 vs. 4.1, P < 0.001). The largest difference in SUVmax was in 2 patients with estrogen receptor–positive/progesterone receptor–positive/human epidermal growth factor receptor 2–negative invasive ductal carcinoma, for whom the highest lesion SUVmax was 23.2 and 17.9 on 68Ga-FAP-2286 PET compared with 7.5 and 7.7 on 18F-FDG PET. 68Ga-FAP-2286 SUVmax was higher in patients with invasive ductal carcinoma (n = 4) than in patients with invasive lobular carcinoma (ILC) or mixed ductolobular carcinoma (n = 5) (SUVmax of 14.6 for invasive ductal carcinoma vs. 4.9 for ILC/mixed; P = 0.046). Anecdotally, the higher uptake on 68Ga-FAP-2286 PET resulted in improved lesion visualization, particularly promising for ILC (Fig. 4).
A 72-y-old woman with metastatic invasive lobular breast cancer. 68Ga-FAP-2286 PET (A) revealed extensive metastatic disease not seen on 18F-FDG PET (B), including small mediastinal and hilar lymph nodes (A, solid black arrow), diffuse gastric mucosal disease (A, dotted black arrow), and extensive peritoneal disease (SUVmax, 7.1; A, black arrowhead and white arrow). Uptake seen on 18F-FDG PET in abdomen reflects physiologic uptake in bowel (B, black arrowhead) rather than tumor.
In total, 5 patients with cholangiocarcinoma and 3 patients with hepatocellular carcinoma (HCC) were enrolled. The SUVmax in cholangiocarcinoma was higher than that in HCC (13.5 vs. 8.6, P = 0.03), although one HCC had an SUVmax of 15.8, overlapping with the cholangiocarcinoma patients. In patients for whom paired 18F-FDG PET was available, both cholangiocarcinoma and HCC had higher uptake on 68Ga-FAP-2286 PET than on 18F-FDG PET (cholangiocarcinoma: 18.1 vs. 9.1, P = 0.009; HCC: 9.3 vs. 4.7, P = 0.04) (Fig. 5). There was no difference in uptake between intrahepatic cholangiocarcinoma lesions and extrahepatic disease (14.0 for intrahepatic vs. 12.7 for extrahepatic, P = 0.7).
A 69-y-old man with cholangiocarcinoma imaged using PET/MRI. (A) 68Ga-FAP-2286 images demonstrate uptake within hepatic metastases. Uptake is more central (solid arrow), which correlates with region of delayed enhancement on MRI (dashed arrow). (B) 18F-FDG PET/CT demonstrates more peripheral uptake associated with more cellular component of tumor (arrow).
DISCUSSION
Here, we report the results of patient imaging using 68Ga-FAP-2286 PET. We evaluated biodistribution and dosimetry, which demonstrated that 68Ga-FAP-2286 is excreted primarily via the kidneys and has a whole-body effective dose consistent with previously studied FAPI agents. Tumor uptake ranged from 5.3 to 16.8 and in general was higher on 68Ga-FAP-2286 PET than on paired 18F-FDG PET, although there was variation among tumor types.
The biodistribution of 68Ga-FAP-2286 is similar to that of previously reported FAPI compounds, with low uptake in all organs except the kidney, consistent with predominate renal excretion. There was a slight increase in liver uptake and kidney uptake compared with the previously reported agents FAPI-04, FAPI-46, and FAPI-72 (11). This is similar to results reported by Pang et al., who also found that the biodistribution was similar between FAP-2286 and FAPI-46, with higher liver and kidney uptake (7). This was also consistent with our dosimetry analysis, which demonstrated a higher absorbed dose in the liver than was seen with 68Ga-FAPI compounds (12,13), supporting greater hepatic accumulation. Overall, the average effective whole-body dose of 68Ga-FAP-2286 PET was 1.16E−02 mSv/MBq, which is slightly lower than that of 18F-FDG PET and similar to that of 68Ga-FAPI-46 (12).
Uptake across tumor types was largely consistent with previous studies of FAPI compounds, including a study by Kratochwil et al. (3). In particular, colon and pancreatic cancer were significantly higher on 68Ga-FAP-2286 PET than on 18F-FDG PET, similar to what was found by Pang et al. (7), which may be attributable to the role of cancer-associated fibroblasts in the desmoplastic reactions surrounding pancreatic cancer cells, as well as the increased presence of fibroblasts in the tumor microenvironment of colon cancer (14,15). In our study, lesions were generally smaller than those reported previously because of cohort 3, which included patients without measurable disease and therefore biased our study toward smaller lesions. The smaller average lesion size could contribute to an underestimate of average SUVmax. This possibility is further supported by the similarity in median uptake between smaller lesions and larger ones. Lesions in the smallest grouping had a median SUVmax of 6.4, compared with 10.3 in the largest, potentially indicating good sensitivity for the detection of small sites of disease.
With head and neck cancer, uptake was greater than seen on 18F-FDG PET, and maybe more importantly, there was anecdotal evidence that inflammatory nodes are not seen on 68Ga-FAP-2286 PET, suggesting that 68Ga-FAP-2286 may have value in nodal staging. Additionally, 68Ga-FAP-2286 might better discriminate between the primary tumor and physiologic uptake in the oropharyngeal mucosa. Consistent with this possibility, previous work has shown that 68Ga-FAPI-04 PET has higher sensitivity and specificity than 18F-FDG PET for nodal metastases (16). In the setting of breast cancer, 68Ga-FAP-2286 PET had higher uptake than 18F-FDG PET, but this was particularly clinically relevant in ILC, for which disease frequently is not seen on 18F-FDG PET (17). Previously, ILC that was 18F-FDG–negative was shown to have uptake on 68Ga-FAP-04 PET (18), and further work should be done in this setting. With liver tumors, there was hope that 68Ga-FAP-2286 PET could help distinguish between HCC and cholangiocarcinoma. Although there was higher uptake in cholangiocarcinoma, there was overlap, which limited the ability of 68Ga-FAP-2286 PET to help characterize intrahepatic lesions, as is consistent with results in prior work (19). One potential benefit of 68Ga-FAP-2286 in cholangiocarcinoma could be the ability to distinguish between an adjacent tumor and inflammation associated with biliary ductal stents and obstruction, which is a common confounder with 18F-FDG PET.
Limitations of our report include the small number of patients enrolled per cancer type, the absence of comparison to 18F-FDG PET in some patients, and the possibility of disease progression within the 3-mo maximum time between 18F-FDG PET and 68Ga-FAP-2286 PET. Future studies with larger populations and smaller imaging windows would improve the robustness of our findings related to 68Ga-FAP-2286 imaging. Additionally, 18F-FDG PET was performed on different scanners, limiting the comparison between the 18F-FDG and 68Ga-FAP-2286 studies.
CONCLUSION
68Ga-FAP-2286 biodistribution, dosimetry, and tumor uptake were similar to those of previously reported FAPI compounds. Additionally, 68Ga-FAP-2286 PET had consistently higher uptake than 18F-FDG PET. These results are especially promising in the setting of small-volume disease and differentiating tumor from inflammatory uptake.
DISCLOSURE
Thomas Hope has grant funding to the institution from Clovis Oncology, GE Healthcare, Lantheus, Janssen, Novartis, the Prostate Cancer Foundation, Telix Pharmaceuticals, and the National Cancer Institute (R01CA235741 and R01CA212148). He received personal fees from Bayer, Cardinal Health, BlueEarth Diagnostics, and Lantheus and received fees from and has an equity interest in RayzeBio and Curium. Robin Kelley has research funding to the institution from Agios, AstraZeneca, Bayer, BMS, Compass Therapeutics, Eli Lilly, EMD Serono, Exelixis, Genentech/Roche, Loxo Oncology, Merck, Novartis, Partner Therapeutics, QED, Relay Therapeutics, Servier, Surface Oncology, Taiho, and Tyra Biosciences. She received consulting/advisory fees from Compass, Exact Sciences, Kinnate, Regeneron, Tyra Therapeutics, and J-Pharma Inc. and to the institution from Agios, AstraZeneca, BMS, Exelixis, Ipsen, and Merck. She received travel support from AstraZeneca and Merck. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What are the dosimetry and tumor uptake of 68Ga-FAP-2286 PET?
PERTINENT FINDINGS: Our prospective study demonstrated a total-body effective dose of 1.16E−02 mSv/MBq and tumor uptake that mirrored that of other FAPI compounds. Additionally, 68Ga-FAP-2286 PET had higher uptake than 18F-FDG PET.
IMPLICATIONS FOR PATIENT CARE: In the setting of small-volume disease and differentiating tumor from inflammatory uptake, 68Ga-FAP-2286 PET could improve staging.
Footnotes
Published online May 2, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 22, 2023.
- Revision received March 25, 2024.