Abstract
This phase 1 study was performed to determine the pharmacokinetics and ability to visualize prostate cancer in bone, soft-tissue, and the prostate gland using 123I-MIP-1072 and 123I-MIP-1095, novel radiolabeled small molecules targeting prostate-specific membrane antigen. Methods: Seven patients with a documented history of prostate cancer by histopathology or radiologic evidence of metastatic disease were intravenously administered 370 MBq (10 mCi) of 123I-MIP-1072 and 123I-MIP-1095 2 wk apart in a crossover trial design. 123I-MIP-1072 was also studied in 6 healthy volunteers. Whole-body planar and SPECT/CT imaging was performed and pharmacokinetics studied over 2–3 d. Target-to-background ratios were calculated. Absorbed radiation doses were estimated using OLINDA/EXM. Results: 123I-MIP-1072 and 123I-MIP-1095 visualized lesions in soft tissue, bone, and the prostate gland within 0.5–1 h after injection, with retention beyond 48 h. Target-to-background ratios from planar images averaged 2:1 at 1 h, 3:1 at 4–24 h, and greater than 10:1 at 4 and 24 h for SPECT/CT. Both agents cleared the blood in a biphasic manner; clearance of 123I-MIP-1072 was approximately 5 times faster. 123I-MIP-1072 was excreted in the urine, with 54% and 74% present by 24 and 72 h, respectively. In contrast, only 7% and 20% of 123I-MIP-1095 had been renally excreted by 24 and 72 h, respectively. Estimated absorbed radiation doses were 0.054 versus 0.110 mGy/MBq for the kidneys and 0.024 versus 0.058 mGy/MBq for the liver, for 123I-MIP-1072 and 123I-MIP-1095, respectively. Conclusion: 123I-MIP-1072 and 123I-MIP-1095 detect lesions in soft tissue, bone, and the prostate gland at as early as 1–4 h. These novel radiolabeled small molecules have excellent pharmacokinetic and pharmacodynamic profiles and warrant further development as diagnostic and potentially when labeled with 131I therapeutic radiopharmaceuticals.
It was estimated that in 2012 there would be 241,740 new cases of prostate cancer diagnosed in the United States and that 28,170 men would die of the disease (1,2). When detected early, and when disease is localized to the prostate gland, the 5-y survival rate is nearly 100%. However, once the cancer has spread beyond the prostate, survival rates fall dramatically (2). Hence the primary goal of staging is to define the anatomic extent of the tumor and to distinguish patients with organ-confined, locally invasive, or metastatic disease. As accurate staging is critical to determining appropriate patient management, it follows that sensitive and specific localization of disease should be a vital component of staging.
Pelvic lymph node dissection represents the most accurate staging procedure for the presence of lymph node invasion in clinically localized prostate cancer (3). However, dissection is invasive and expensive and may be associated with significant morbidity. CT and MR imaging are noninvasive procedures that are commonly used for nodal staging. However, a recent meta-analysis of the diagnostic accuracy of presurgical CT or MR imaging for staging pelvic lymph nodes suggests that the value of these modalities in the staging of prostate cancer may be low (4). The low sensitivity of both techniques suggests that CT or MR imaging will likely misrepresent the patient’s true status regarding nodal metastases and could lead to a suboptimal therapeutic approach (4).
Early detection of bone metastases is critical in the management of patients with high-risk prostate cancer. Newly diagnosed patients with localized disease and no metastases may benefit from radical treatment with curative intent. In contrast, most guidelines recognize that patients with bone metastases should forgo local therapy to avoid unnecessary side effects and be treated with systemic therapy instead (5). Early metastases to bone may be missed with bone scanning because this technique relies on osteoblastic activity (mineralization) rather than detection of actual tumor cells (6). A true and sensitive measure of the presence of disease in bone should lead to earlier detection of metastatic spread and a more robust measure of changes in tumor burden that could guide patient management.
Although current imaging techniques have limitations in the diagnosis and staging of prostate cancer, new imaging approaches that can more accurately assess the status of the disease will facilitate the selection of optimal treatment and improve patient outcomes. One strategy is to use radiopharmaceuticals that specifically target an upregulated enzyme on the surface of prostate cancer cells such as prostate-specific membrane antigen (PSMA). PSMA, also known as folate hydrolase I or glutamate carboxypeptidase II, is a transmembrane 750-amino-acid type II glycoprotein that is primarily expressed in normal human prostate epithelium but is overexpressed in prostate cancer (7), including metastatic disease (8,9). Because PSMA is expressed by virtually all prostate cancers and its expression is further increased in poorly differentiated, metastatic, and hormone-refractory carcinomas (10,11), it is an attractive target for prostate cancer imaging and therapy. Currently, a radiolabeled anti-PSMA antibody (ProstaScint [capromab pendetide]; EUSA Pharma.) is approved to detect soft-tissue metastasis and recurrence of prostate cancer. However, this antibody targets the intracellular domain of PSMA and is thought to bind mostly to necrotic cells of prostate tumors (12). Clinical trial results with the monoclonal anti-PSMA antibody J591 have shown PSMA to be a useful diagnostic and therapeutic target (13,14). Although antibodies offer potential for tumor targeting, their effectiveness as diagnostic radiopharmaceuticals is limited by a long circulating half-life and poor tumor penetrability, particularly for bone metastases.
Several investigators have reported on the use of small-molecule inhibitors of PSMA labeled with 123I (15–17), 99mTc (18,19), 18F (20), 111In (21), and 68Ga (22) with potential for imaging PSMA. In the present study, two high-affinity radioiodinated PSMA inhibitors described previously (16,17), 123I-MIP-1072 and 123I-MIP-1095, were studied in prostate cancer patients and healthy volunteers under an exploratory investigational new drug (IND) application. The aims of these early phase 1 investigations were to assess the tumor-localizing ability, pharmacokinetics, organ-absorbed radiation dose, and excretion of 123I-MIP-1072 and 123I-MIP-1095.
MATERIALS AND METHODS
Study Design
All procedures described in the following studies were approved by the institutional review boards of the participating medical institutions. The first study was an open-label crossover design of 2 novel study drugs, 123I-MIP-1072 and 123I-MIP-1095, involving 7 patients with a documented history of prostate cancer by histopathology or radiologic evidence of metastatic disease with a rising level of PSA. In 1 case, metastatic disease could not be confirmed with standard radiographic imaging techniques. The sequence of study drugs was randomized. Patients with metastatic prostate cancer were invited to participate, were screened for eligibility, and provided written informed consent. After undergoing baseline examinations, the patients received the first study drug and were followed for 72 h to gather whole-body planar γ-camera images, blood and urine samples for assay of radioactivity, and SPECT/CT of the pelvis. On completion of a 2-wk washout period, the same patients were administered the second study drug and underwent the same evaluation as for the first drug. They were followed from enrollment through 2 wk after the second test article administration to collect safety data. In the second study, 123I-MIP-1072 was administered to 6 healthy volunteers to evaluate pharmacokinetics, tissue distribution, excretion, safety, and organ radiation dose with a focus on uptake in the pelvis using SPECT/CT.
Study Drugs
The synthesis of MIP-1072 [(S)-2-(3-((S)-1-carboxy-5-(4-iodobenzylamino)pentyl)ureido) pentanedioic acid] and MIP-1095 [(S)-2-(3-((S)-1-carboxy-5-(3-(4-iodophenyl)ureido)pentyl)ureido)pentanedioic acid] and radiolabeling with 123I were described previously (16,17) and were performed under good-manufacturing-practice conditions. The structure and calculated logP values of MIP-1072 and MIP-1095 are shown in Figure 1.
Chemical structure and calculated logP values (cLogP) of MIP-1072 and MIP-1095.
123I-MIP-1072 and 123I-MIP-1095 Administration
In the first study, each patient was randomly assigned to receive 370 MBq (10 mCi) of either 123I-MIP-1072 or 123I-MIP-1095 intravenously followed 2 wk later by the second compound. The 370 MBq dose was required to obtain robust count statistics for accurately characterizing the systemic distribution of 123I as a function of time. In the second study, healthy volunteers were intravenously administered 370 MBq of 123I-MIP-1072. The radioactive administered dose was determined by measuring the amount of radioactivity in the syringe before and after administration with a radioisotope dose calibrator. Thyroidal uptake was blocked at 2 of the 3 study sites (4/7 patients) using inorganic iodide. In the healthy volunteer study, thyroidal blockade was not performed.
Image Acquisition
Whole-body scintigraphy was performed at 0.5, 1, 2, 4–6, 24, and 48 h after injection and, in prostate cancer patients, at 72 h. Anterior and posterior planar projections were acquired using dual-head γ-cameras equipped with low-energy high-resolution collimators. Scan velocity was incrementally slowed from 15 cm/min at 30 min and 1 h after injection to 10 cm/min at 2 h and 4–6 h after injection and then 3–5 cm/min for all remaining time points. A matrix size of 256 × 1,024 pixels and a symmetric window of 15% centered on a 159-keV photopeak were used for all acquisitions. A single imaging standard of approximately 18.5 MBq (500 μCi) was placed in the field of view for each whole-body acquisition to allow for decay and scan speed correction across all time points.
Tomographic SPECT of the abdomen and pelvis was performed at approximately 4 and 24 h after injection. When available, SPECT/CT was performed to obtain measured attenuation correction maps and to aid in anatomic localization using standard low-dose CT parameters. SPECT parameters consisted of a 360° rotation, 30 s per stop, and a 3° azimuth using a step-and-shoot technique. A matrix size of 128 × 128 and a symmetric window of 15% centered on a 159-keV photopeak were used for all SPECT acquisitions. Raw SPECT data were reconstructed into 3-dimensional volumes and corrected for attenuation on a workstation (Hermes Medical Solutions) using ordered-subsets expectation maximization iterative reconstruction methods (3 iterations and 30 subsets) and filtered with a Butterworth filter (cutoff, 1.2; order, 5). Standard-of-care diagnostic CT of the chest, abdomen, and pelvis was obtained in patients with known prostate disease and registered to SPECT studies if a SPECT/CT scan was not acquired.
Image Analysis
Regions of interest (ROIs) were created over the imaging standard, tissues, and normal organs on each anterior and posterior planar whole-body projection to extract count data corresponding to 123I-MIP-1072 and 123I-MIP-1095 uptake. The mean count for each ROI at the first post-dose image was divided by the mean count for the whole-body ROI at the first post-dose image. The resulting ratio is expressed as a percentage and referred to as the percentage of injected activity for that organ at the initial time point. Because of poor counting statistics at the 72-h time point (∼6 half-lives), image analysis was performed on images acquired up to 48 h after injection. Tissue kinetic and mean residence time data can be found in supplemental material (available online at http://jnm.snmjournals.org).
Additional analyses of target-to-background count ratios were performed on planar and reconstructed SPECT images. For planar images, counts were obtained from fixed 4 × 4 pixel ROIs placed over target lesions identified by 2 independent readers and immediately adjacent to the lesion as a background. Target-to-background ratios for lesions within the SPECT field of view were calculated by obtaining counts from a circular 10-pixel ROI in the axial projection and an equal-sized ROI placed immediately adjacent as the background. Axial SPECT slices were also used to obtain prostate counts using a registered CT scan for proper anatomic localization within the prostate gland. A rectangular 20-pixel ROI was placed over the gland and immediately to the left to obtain prostate-to-background counts in patients with an intact prostate gland.
Pharmacokinetics and Excretion
Blood samples were collected from prostate cancer patients at 2–15 min and 1, 2, 4, 6, 24, 48, and 72 h after injection of 123I-MIP-1072 or 123I-MIP-1095; urine was collected and pooled at time intervals of 0–4, 4–24, 24–48, and 48–72 h after dosing. In the healthy volunteer study, blood and urine were collected only through 48 h after injection using the same time intervals. Blood and urine samples were analyzed for 123I activity using γ-well counting. The pharmacokinetic data were analyzed with WinNonlin software, version 4.1 (Pharsight). Blood and tissue concentration–time data were computed by noncompartmental analysis (WinNonlin model 201), and pharmacokinetic parameters, such as Cmax and mean residence time, were generated. Total clearance was calculated as dose divided by area under the curve (AUC), and steady-state volume of distribution was calculated as the product of total clearance and mean residence time. Distribution half-life and elimination half-life were obtained by fitting the 2-compartment model with bolus input and first-order output (WinNonlin model 7). The rate of urinary clearance and the 123I activity recovered in the urine were also computed by noncompartmental analysis (WinNonlin model 211).
Organ-Absorbed Radiation Dose Estimates
The OLINDA/EXM software was used to estimate absorbed radiation dose to target organs (23,24). The adult male model was used for all patients. The urinary bladder was assumed to be voided regularly at 4- to 8-h intervals, and the gastrointestinal transit times of the human adult male were assumed. The small-bowel and upper and lower large-intestine residence times were computed using the provided implementation of the gut transit model of publication 30 from the International Commission on Radiological Protection. The radiation doses to salivary glands and tumors were determined using spheric S values as implemented in OLINDA/EXM.
Safety
Safety data were collected from treatment-emergent adverse event reports, pre- versus postinjection electrocardiograms, physical examinations, vital signs, and laboratory measurements (including clinical chemistry, hematology, and urinalysis). Adverse events were coded by the Medical Dictionary for Regulatory Activities and were summarized by occurrences and percentages of patients according to grade (National Cancer Institute Common Terminology Criteria), body system, preferred term, intensity, and causal relationship to study agent. SAS software (version 9.1; SAS Institute) was used for the safety analysis.
RESULTS
Patients
A summary of the diagnosis and treatment history for each patient is shown in Table 1. All 7 patients had a documented history of prostate cancer by histopathology. Six of 7 patients had radiologic evidence of metastatic disease with rising PSA. The first study consisted of 7 men ranging in age from 53 to 86 y with a mean PSA of 121 ng/mL, 6 of whom were hormone-refractory. Three of the 7 patients had intact prostate glands. The second study consisted of 6 healthy male volunteers ranging in age from 23 to 61 y with intact prostate glands and baseline PSA levels in the reference range.
Summary of Diagnosis and Treatment History for Prostate Cancer Patients
Imaging Studies and Tissue Distribution
Typical whole-body planar and SPECT images for a patient administered 123I-MIP-1072 and 123I-MIP-1095 through 24 h after injection are depicted in Figure 2. Within 0.5–1 h after injection, metastatic bone and lymph node lesions could be detected. Uptake was also seen in the liver, bowel, kidneys, salivary glands, parotid glands, and lachrymal glands. Both the kidneys and the salivary glands are reported to express PSMA (25,26). The signal in the kidneys is likely a combination of target binding and renal clearance. As the blood and soft-tissue background cleared, lesion-to-background ratios derived from whole-body planar images increased from an average value of 2:1 at 1 h to 3:1 at 4 and 24 h after injection for 123I-MIP-1072 and 123I-MIP-1095 (Fig. 3A) but were greater than 10:1 at 4 and 24 h when using SPECT (Fig. 3B).
Representative anterior whole-body planar (left) and SPECT/CT (right) images of 123I-MIP-1072 and 123I-MIP-1095 administered at 370 MBq (10 mCi) to patient with radiographically confirmed metastatic prostate cancer. Planar images are through 24 h after injection. Image intensity was normalized to first image using reference standard next to right leg in each image. SPECT/CT images are at 4 h after injection. Arrows depict localization of both compounds in confirmed lesions in paraaortic lymph nodes (LN) and bone (Bn) of lumbar spine.
Summary of target-to-background ratios derived from whole-body planar (A) and SPECT (B) images for patients with metastatic prostate cancer. Patients were administered 370 MBq (10 mCi) of 123I-MIP-1072 (solid bar) and 123I-MIP-1095 (open bar) in crossover design. Each histogram is mean ± SEM for 6 patients.
123I-MIP-1072 was cleared from the body more rapidly than 123I-MIP-1095. At 6 h after injection 62% ± 5% of the injected dose remained in the body for 123I-MIP-1072, and by 48 h after injection 30% ± 5% of the injected dose remained in the body. In contrast, 123I-MIP-1095 was cleared from the body more slowly, resulting in increased soft-tissue uptake. At 6 h after injection 98% ± 2% of the injected dose was still resident in the body for 123I-MIP-1095, and by 48 h after injection 85% ± 4% of the injected dose remained in the body. In all organs, approximately 2 times greater retention was observed with 123I-MIP-1095, with a concomitant increase in tumor uptake at similar ratios.
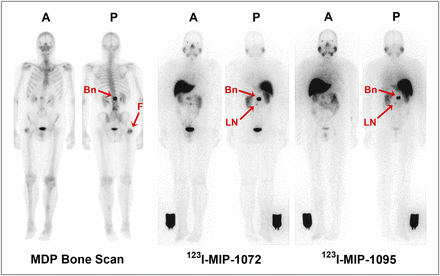
Rapid uptake into the lesions present in the bone, lymph nodes, and prostate gland was observed with both agents. As was observed in mouse xenograft studies (17), both agents were retained in the tumors throughout the study period, with increasing uptake observed for 123I-MIP-1095 through 24 h after injection whereas nontarget tissues were more rapidly cleared with 123I-MIP-1072. The lesion-to-background ratio was slightly higher for 123I-MIP-1072 than for 123I-MIP-1095, resulting from the slower total-body clearance and greater lesion uptake observed with 123I-MIP-1095 (Figs. 3A and 3B). However, both agents had sufficient lesion uptake and background clearance to permit detection of lesions in the bone (as confirmed by bone scanning) and enlarged lymph nodes (Fig. 4) consistent with radiologic studies. Uptake in the head of the femur on bone scintigraphy (Fig. 4), likely due to degenerative changes, was not detected with either 123I-MIP-1072 or 123I-MIP-1095. Using SPECT/CT, both agents were able to detect lesions in the prostate gland at 4 and 24 h after injection (Fig. 5). In healthy volunteers 123I-MIP-1072 was cleared rapidly in a similar fashion to that observed in prostate cancer patients. Low uptake was observed in the prostate gland in healthy volunteers, a finding that supports the potential for visualization of disease in the prostate by SPECT/CT (Fig. 5).
Representative anterior (A) and posterior (P) whole-body planar images of patient with radiographically confirmed metastatic prostate cancer who received 740 MBq (20 mCi) of 99mTc-methylene diphosphonate (MDP) (left), followed by 123I-MIP-1072 (middle) and 123I-MIP-1095 (right) administered at 370 MBq (10 mCi). Depicted are images acquired at 4 h after injection. Arrows indicate detection of confirmed lesions in bone (Bn) of lumbar spine and uptake in suggestive 7-mm lymph node (LN). Image reference standard was placed next to right leg. Subject had previous hip replacement as demonstrated by uptake in head of right femur (F) only on bone scan.
Patients with radiographically confirmed metastatic prostate cancer to whom 370 MBq (10 mCi) of 123I-MIP 1072 and 123I-MIP-1095 were administered, and for comparative purposes, healthy volunteer to whom 370 MBq of 123I-MIP 1072 were administered. Depicted are representative transaxial (left), coronal (middle), and sagittal (right) slices from reconstructed SPECT/CT at 4 h after injection.
Pharmacokinetics and Excretion
After intravenous administration, blood levels of 123I-MIP-1072 and 123I-MIP-1095 declined in a biphasic fashion (Fig. 6A). 123I-MIP-1072 was rapidly cleared from the vascular compartment and moved into the extravascular space, resulting in a total-blood exposure (AUC, 0–infinity) of 0.13 ± 0.01 h⋅MBq/mL (3.5 ± 0.3 h⋅μCi/mL) and a mean residence time of 24.1 ± 4.8 h. 123I-MIP-1072 was cleared predominantly via the kidney, with the most rapid rate observed during the first 4 h after injection. By 24 h after injection, 54% ± 3% of the injected radioactivity was present in the urine (Fig. 6B). At the conclusion of the 72-h postinjection monitoring period, 74.3% ± 3% of the injected radioactivity was excreted in the urine. Similar blood pharmacokinetic and clearance results were observed in healthy volunteers administered 123I-MIP-1072; at the last collection time point (48 h), 59% ± 6% of the injected radioactivity was excreted in the urine (Fig. 6B). Feces were not collected.
Blood clearance (A) and cumulative percentage urine recovery (B) of 123I-MIP 1072 (squares) and 123I-MIP 1095 (circles) administered at 370 MBq (10 mCi) to 7 prostate cancer patients using randomized crossover design and 123I-MIP-1072 (triangles) administered at 370 MBq to 6 healthy volunteers. Depicted is mean radioactivity present in blood expressed as μCi/mL ± SEM graphed from 0.001 μCi/mL (37 Bq) to 10 μCi/mL (0.37 MBq), and cumulative recovery in urine expressed as percentage injected dose over time for each study group.
123I-MIP-1095 was cleared from the vascular compartment and moved into the extravascular space more slowly than 123I-MIP-1072, resulting in a total-blood exposure (AUC, 0–infinity) for 123I-MIP-1095 of 0.57 ± 0.1 h⋅MBq/mL (15.3 ± 2.8 h⋅μCi/mL) and a mean residence time of 56.9 ± 12.7 h. 123I-MIP-1095 was minimally cleared by the kidneys, with only 7.5% ± 4.7% of the injected radioactivity present in the urine at 24 h and 20.3% ± 8.7% of the injected radioactivity excreted in the urine at 72 h after injection (Fig. 6B). Feces were not collected.
Radiation Dosimetry
The absorbed radiation dose for organs was estimated in units of mGy/MBq. The complete radiation dose estimates (a standard list of 25 organs, salivary glands, total body, and effective dose and effective dose equivalent) for all subjects were used to generate the descriptive statistics shown in Table 2, with organs sorted in descending order of mean radiation dose for 123I-MIP-1072. For 123I-MIP-1072, the organ-absorbed radiation doses to the urinary bladder wall (assuming a 4.8-h void), salivary glands, and kidneys were the largest. No organ radiation dose exceeded 0.1 mGy/MBq (Table 2). For 123I-MIP-1095, the absorbed doses to the salivary glands, kidneys, and thyroid were the largest. Other than these 3 organs, no mean organ-absorbed dose exceeded 0.1 mGy/MBq (Table 2). Thyroid blockade (4/7 patients) was effective at lowering the absorbed dose to the thyroid with both compounds. 123I-MIP-1095 delivered substantially higher thyroid doses than did 123I-MIP-1072, regardless of whether blockade was used. 123I-MIP-1072 exhibited more than a 50% decrease in thyroid-absorbed radiation dose when blockade was used (0.050 vs. 0.030 mGy/MBq), whereas 123I-MIP-1095 decreased from 0.120 to 0.084 mGy/MBq. The estimated organ-absorbed radiation dose for 123I-MIP-1072 was similar in both prostate cancer patients and healthy male volunteers. In the healthy volunteers, the thyroid dose was slightly higher since a pretreatment thyroid blockade regimen was not performed. Overall, the 123I-MIP-1072 organ-absorbed radiation doses were about 30%–60% lower than those of 123I-MIP-1095, except for the bladder wall. However, bladder wall absorbed dose was 4.4-fold greater for 123I-MIP-1072 because of its more complete and rapid urinary clearance (Table 2). The bladder dose can be substantially reduced by encouraging consumption of fluids and more frequent voiding.
Estimates of Mean Absorbed Radiation Dose in Prostate Cancer Patients and Healthy Volunteers
Safety
Overall, both 123I-MIP-1072 and 123I-MIP-1095 were well tolerated in this study population. Of the 7 patients receiving both 123I-MIP-1072 and 123I-MIP-1095, 3 patients reported a total of 4 treatment-emergent adverse events, all of which were mild (grade 1). The adverse events consisted of chills, constipation, insomnia, and mild injection site irritation, and all resolved without treatment. No treatment-emergent adverse events were observed in healthy volunteers receiving 123I-MIP-1072. Clinical laboratory results and physical examinations revealed no clinically relevant changes from the pre-dose to post-dose study period in prostate cancer patients or healthy volunteers.
DISCUSSION
Using the exploratory IND approach, we were able to compare the pharmacokinetics, tissue distribution, excretion, and metastatic lesion localization of 2 structurally related small molecules, 123I-MIP-1072 and 123I-MIP-1095, to select, if suitable, a candidate for further clinical development. Both agents were based on the glutamate-urea-lysine moiety modified with iodine containing aromatic substituents at the ɛ-amine of lysine, and both radiolabeled compounds were shown previously to bind with high affinity to the extracellular enzymatic domain of PSMA on prostate cancer cells (16,17).
In designing an ideal imaging agent for a specific cancer, there are particular characteristics that must be considered. It is important to select a molecular target that is specific to the cancer in question and one that has consistently high expression levels per tumor cell throughout the natural progression of the disease, and ideally throughout therapy. This is particularly critical for assessing the presence of disease and changes in tumor mass and spread, whether it is low-grade localized disease or response to treatment in a patient with advanced-stage metastatic disease (27). In prostate cancer, the ability to visualize true disease burden in bone could lead to a more robust measure of changes in tumor burden that is clinically relevant to patient management. Likewise, visualization of the presence of tumor in lymph nodes that are normal by anatomic criteria would be a significant improvement in the current state of the art and lead to improved staging.
PSMA was chosen as the target since it is endogenously expressed at low levels in normal prostate, brain, kidney proximal tubules, and intestinal brush border membranes (10,25,26,28). Reports indicate that increased expression of PSMA in primary prostate cancer correlates with other adverse traditional prognostic factors and independently predicts disease outcome (11,29,30). Importantly, expression of PSMA is dramatically upregulated in poorly differentiated, metastatic, and hormone-refractory carcinomas (11), as well as after androgen deprivation therapy (31) and in lymph node metastases (32). The results of the present study demonstrate that targeting PSMA with both 123I-MIP-1072 and 123I-MIP-1095 facilitates the detection of radiologically proven prostate cancer in bone, lymph nodes, and the prostate gland.
Ligand selection should be based on rapid uptake and persistent localization at the target site with minimal retention in nontarget tissue. Small molecules have a decisive advantage over much larger proteins given their faster rate of clearance from the blood and increased tumor permeability, which allow them to evade physiologic barriers encountered by larger molecules such as antibodies. On the basis of the pharmacokinetic parameters obtained from this study, both compounds display rapid distribution phases and blood clearance rates, with 123I-MIP-1072 being cleared from the circulation and nontarget tissues more quickly. In parallel, both compounds show rapid tumor uptake, resulting in excellent tumor–to–normal-tissue contrast on planar and SPECT scintigraphy as demonstrated in Figures 2, 4, and 5.
A particular advantage of the exploratory IND is to rapidly bring similar compounds into the clinic to confirm preclinical data. Although 123I-MIP-1072 and 123I-MIP-1095 are based on the same amino acid heterodimeric core and display similar PSMA binding affinities, significantly different pharmacokinetic parameters were observed, most likely because of modest structural differences affecting hydrophobicity and protein binding. In the case of these 2 candidate molecules, target avidity is maintained while blood clearance and normal-organ clearance rates differ significantly. Such differences are seen predominately in the AUC for blood, with the more hydrophobic compound, 123I-MIP-1095, having approximately a 4-fold increase in AUC compared with 123I-MIP-1072. In addition, there is on average greater absolute tumor uptake with 123I-MIP-1095, although, due to nontarget tissue retention as a result of slower whole-body clearance, the signal-to-noise ratios for 123I-MIP-1072 are more attractive during the earlier time period and most practical for a diagnostic agent. Greater tumor uptake of 123I-MIP-1095 than of 123I-MIP-1072 in xenografts, coupled with prolonged elimination of 123I-MIP-1095 through a combination of renal and gastrointestinal excretion as opposed to clearance predominantly in the urine for 123I-MIP-1072, was also observed in rodent studies.
Since PSMA has been shown to be endogenously expressed in the normal prostate bed at low levels (25,33), the tissue distribution, whole-body clearance, and retention of 123I-MIP-1072 was also studied in healthy volunteers. On SPECT, low uptake of PSMA-targeting 123I-MIP-1072 was observed in the normal prostate gland (target-to-background ratio, ∼2:1) whereas 3–5 times greater uptake was seen in the prostate gland in prostate cancer patients. In view of these findings, further investigation is warranted in patients to determine whether imaging intraprostatic cancer is possible.
In the present study, both 123I-MIP-1072 and 123I-MIP-1095 localized to lesions in bone and soft tissue that correlated with radiologic evidence of metastatic disease. However, in several patients significant uptake was also observed, using planar whole-body and SPECT imaging, in lymph nodes smaller than 10 mm, considered normal by size criteria used in cross-sectional imaging such as CT and MR. Although histologic confirmation of disease in the lymph nodes is needed, these observations suggest an improvement in the sensitivity of lesion detection with molecular imaging using 123I-MIP-1072 and 123I-MIP-1095 and that perhaps the future state of the art will be some combination of molecular targeting agents such as 123I-MIP-1072 and 123I-MIP-1095 and anatomic imaging.
CONCLUSION
Microdosing studies under an exploratory IND allowed for the assessment of preliminary pharmacokinetics and pharmacodynamics for two radioiodinated small molecules, 123I-MIP-1072 and 123I-MIP-1095, that selectively bind to PSMA with high affinity. Both compounds localized to lesions in bone and soft tissue that correlated with radiologic evidence of metastatic prostate cancer. Minimal uptake of one of these compounds (123I-MIP-1072) was seen in the prostate gland of healthy volunteers, suggesting the possibility of visualizing disease in that organ. On the basis of the higher signal-to-background ratio, 123I-MIP-1072 was evaluated as a diagnostic agent in subsequent clinical trials, and on the basis of the prolonged tumor retention as a result of slower tissue kinetics, 131I-MIP-1095 will be evaluated clinically for radiotherapy. Hence, in this case the exploratory IND has afforded the opportunity to select both diagnostic and therapeutic radiopharmaceuticals.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. No potential conflict of interest relevant to this article was reported.
Footnotes
↵† Deceased.
Published online Jan. 9, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication July 13, 2012.
- Accepted for publication October 1, 2012.