The success of targeting and exploiting the prostate-specific membrane antigen (PSMA) for diagnosis and radioligand therapy of prostate cancer has been remarkable. Unlike in early-phase drug trials, investments by industry have been minimal. Nevertheless, the few institutions in the United States that currently offer PSMA theranostics have difficulty meeting the demand. Invented by various groups of researchers (1–7), PSMA theranostics has found success largely through the efforts of patients and their treating physicians.
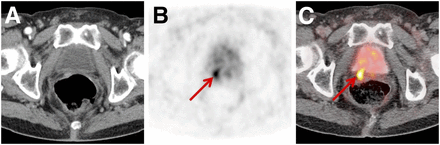
Two reasons account for the rapid clinical adoption of PSMA-targeted theranostics. First, there is a highly relevant unmet clinical need for detecting sites of recurrence early, when salvage therapy can still be curative (8). 68Ga- or 18F-labeled PSMA ligand imaging detects sites of recurrence in more than 80% of all patients (9–13) and even in 90% of patients after external-beam radiotherapy or brachytherapy (Fig. 1) (14). The data were summarized in a recent metaanalysis that also highlighted a potential value in very early recurrent disease (prostate-specific antigen level < 0.2 ng/mL) (15), and another published study found that 68Ga-PSMA ligand imaging clearly outperformed 18F-fluoromethylcholine imaging (16). The available data attest a high accuracy for N-staging and high detection rates for primary and recurrent prostate cancer (15). Thus, a recent expert position paper has already favored it over other techniques, especially in the setting of early biochemical recurrence (17).
CT image (A) and 68Ga-PSMA-11 PET (B) and PET/CT (C) images of 70-y-old man after radical prostatectomy, biochemical recurrence (prostate-specific antigen level, 0.68 ng/mL), and negative results on conventional imaging. Intense 68Ga-PSMA-11 ligand uptake (arrow) dorsal to bladder in small soft-tissue mass clearly indicates local recurrence.
Second, PSMA serves as a highly relevant therapeutic target. Therefore, imaging of its expression provides a uniquely successful example of a predictive imaging biomarker. Patients are stratified by PSMA ligand imaging for PSMA-targeted therapy using ligands labeled with 177Lu (18) or 225Ac (19). The reported efficacy is extremely promising (18,20,21). For example, in a recent multicenter retrospective analysis of over 100 patients being treated with 177Lu-PSMA, more than 40% showed a greater than 50% decline in prostate-specific antigen levels (18)—a typical intermediate endpoint for treatment studies on metastatic prostate cancer. Hence, solid data on a potential improvement in overall survival compared with a standard treatment scheme are lacking, but preliminary reports are highly encouraging (21,22).
The question to be posed is whether a commonsense approach can be adopted to facilitate a rapid approval and reimbursement process for the 68Ga/177Lu-PSMA theranostic pair. Drug approval, from target identification to clinic, frequently takes as long as 10 y, and radioactive-drug approval is often not much faster. For example, peptide receptor radionuclide therapy for neuroendocrine tumors was introduced in the 1990s but still is not approved in the United States.
How can we expedite the regulatory processes to make PSMA theranostics available to the hundreds of thousands of patients who could benefit? The Food and Drug Administration has been receptive to efforts led by the Clinical Trials Network of the Society of Nuclear Medicine and Molecular Imaging and has granted Investigational New Drug applications with cost recovery for imaging and, just recently, for therapeutic trials. This mechanism allows patients to be charged for imaging and therapeutic applications—a solution that is, however, only temporary as well as ethically questionable, since only patients who can afford the cost will be able to participate in a study.
We suggest an unusual solution—namely a commonsense approach—for this problem that takes into account the following 5 considerations:
First, consider that PET/CT imaging with any imaging probe is safe. Millions of 18F-FDG PET/CT scans have been performed, and not a single significant side effect has been reported. 68Ga- or 18F-PSMA ligand PET/CT is also safe because the imaging probe, given in nanomolar concentrations, cannot have any effects or side effects. Thousands of patients have been imaged and not a single significant side effect has been reported.
Second, consider that medical radiation from diagnostic imaging has no known risks (23).
Third, consider that PSMA ligand imaging is effective. Sites of recurrent disease are detected at low levels of serum prostate-specific antigen, and PSMA imaging is able to stage disease more accurately than any other tool. The impact on management has been documented prospectively (24) and retrospectively (25).
The fourth consideration is that although even now there are ample safety and toxicity data for the 177Lu-labeled therapeutic compound, initial studies under Investigational New Drug applications will soon provide more than enough additional data to justify Food and Drug Administration approval.
The final consideration is that the exceptionally high demand for PSMA-targeted theranostics is providing the strongest evidence of its usefulness. Neither patients nor treating physicians have a conflict of interest, financial or otherwise, nor can they be fooled for long by meritless hype. Rather, they are apparently already convinced by their experience with PSMA-targeted theranostics. Why can we not consider the market as the strongest and most reliable indicator of usefulness? More than 2,000 PET probes have been created, and only a handful have made it to the clinic. This high failure rate is likely due to the possibility that most probes did not address an urgent unmet need. PSMA-targeted theranostics is obviously different. Its adoption is driven by the market—that is, patients and their treating physicians—because it successfully addresses an unmet need.
In conclusion, as this theranostic approach is safe and effective, it should be expeditiously approved by the Food and Drug Administration once prospective data on toxicity and patient safety are available. Reimbursement by the Centers for Medicare and Medicaid Services should also be rapid, as the market will quickly decide whether the approach is useful. In the extremely unlikely scenario that the enthusiasm and thus market demand for this theranostic approach vanishes, it will simply disappear. Thus, the risk to taxpayers is minimal.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online May 4, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication March 20, 2017.
- Accepted for publication April 26, 2017.