Abstract
Radiolabeling of the prostate-specific membrane antigen (PSMA) inhibitor Glu-NH-CO-NH-Lys(Ahx) using the 68Ga chelator HBED-CC (PSMAHBED) allows imaging of prostate cancer lesions because of high expression of PSMA in prostate carcinoma cells and in bone metastases and lymph nodes related to the disease. The aim of this work was to optimize labeling of 68Ga-PSMAHBED using the efficient cation-exchange postprocessing of 68Ga as well as the development of a thin-layer chromatography (TLC)–based quality control system. Methods: Labeling was optimized for online ethanol-postprocessed 68Ga eluate investigating various parameters, such as buffer molarity (0.1–1 M), temperature (25°C–90°C), tracer amount (0.11–0.74 nmol), and labeling time. In addition, purification of the crude product was tested. For radio-TLC quality control, various mobile phases were analyzed using silica gel 60 plates and the results were validated using high-performance liquid chromatography. The most superior mobile phases were also applied on instant thin-layer chromatography (ITLC) silica gel plates. Results: Using optimized conditions, labeling yields of more than 95% were obtained within 10 min when ethanol-based postprocessing was applied using PSMAHBED amounts as low as 0.1 nmol. A higher precursor concentration (0.7 nmol) further increased labeling and quantitative yields to more than 98% within 5 min. In clinical routine, patient batches (>200 applications) with radiochemical purity greater than 98% and specific activities of 326 ± 20 MBq/nmol are obtained reproducibly. When TLC quality control was performed on silica gel 60 plates, 4 mobile phases with suitable separation properties and complementary Rf values were identified. Two systems showed equivalent separation on ITLC silica gel plates, with ITLC analysis finished within 5 min, in contrast to 20 min for the TLC system. Labeling of PSMAHBED was optimized for cation-exchange postprocessing methods, ensuring almost quantitative labeling and high nuclide purity of final 68Ga-PSMAHBED, making subsequent purification steps unnecessary. Conclusion: The new radio-TLC method allows quality control in a short time using a fast, reliable, low-cost method with little equipment complexity. Using this approach, the synthesis is easily adopted by automated synthesis modules.
Prostate-specific membrane antigen (PSMA) is a cell surface protein with increased expression on nearly all prostate cancer cells compared with other PSMA-expressing tissues such as kidney, proximal small intestine, or salivary gland (1–3). Because PSMA expression is restricted to the prostate and the cell surface at all stages of disease, it holds promise as a target for specific imaging and therapy of prostate cancer and neovasculature (4–6). Studies have recently shown that low-molecular-weight peptidomimetic radiopharmaceuticals are clinically attractive because prostate cancer lesions can be imaged with high contrast and higher sensitivity than is possible with 18F-choline-PET/CT (7–10). Noninvasive imaging of increased PSMA expression provides important information on the stage of prostate cancer and the location of metastatic lesions.
One of those peptidomimetic radiopharmaceuticals is Glu-NH-CO-NH-Lys(Ahx)-HBED-CC (PSMAHBED, PSMA-11), showing high potential as a prostate cancer imaging agent (1,8). It is a urea-based PSMA inhibitor including the acyclic complex ligand N,N′-bis[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid (HBED-CC), being recently proposed as a chelator for efficient radiolabeling with generator-produced 68Ga at room temperature (11). In addition, the lipophilic character of the 68Ga complex of HBED-CC was found to be a necessary feature for interaction with the PSMA binding site (1,12,13).
Generator-produced 68Ga represents an attractive alternative to cyclotron-based PET nuclides such as 18F or 11C but requires protocols to provide 68Ga suitable for medical use. Several methods have been developed for purification of 68Ga eluate to fulfill regulatory requirements (13–15). Initial publications on 68Ga-PSMAHBED used crude 68Ga generator eluate for 68Ga labeling of PSMA and high-performance liquid chromatography (HPLC) for quality control. This report describes radiolabeling of PSMAHBED using cation-exchange–based postprocessing methods for manual synthesis as well as the use of an automated module followed by the development of a thin-layer chromatography (TLC)– and instant TLC (ITLC)–based quality control system.
MATERIALS AND METHODS
Only the highest-reagent-grade chemicals and TraceSELECT water were used. Chemicals were purchased from Sigma Aldrich and used without further purification, unless stated otherwise. 68Ga was obtained from an initially 1.1-GBq 68Ge/68Ga generator (2 y old) from Cyclotron Co. Ltd. and from an initially 1.85-GBq 68Ge/68Ga generator (new) from iThemba Labs.
BioRad AG 50W-X4 (200–400 mesh) cation-exchange resin was used to prepare a microchromatography column (50 mg of resin, 2-mm inner diameter, 5-mm length). Also, Varian Bond Elut-SCX was used. Labeling reactions were performed in 11-mL glass vials (Mallinckrodt) using a block thermostat (TK13; Ditabis) for temperature control and agitation. Purification was performed with 30-mg C-18 cartridges (Phenomenex Strata-X Tubes). Activity was measured using a curie meter (ISOMED 2010; Nuklear-Medizintechnik Dresden GmbH). pH was measured using a calibrated pH meter (SevenEasy pH; Mettler-Toledo). TLC plates (aluminum-backed silica gel 60; Merck) and ITLC silica gel plates (Varian) were analyzed using a flat-bed scanner (Instant Imager [Canberra Packard] or miniGita [Raytest-Isotopenmessgeräte GmbH]).
Reverse-phase HPLC using a LiChrosphere 100-RP18EC column (5 mm, 250 × 4 mm) was applied to quantify the radiochemical purity of 68Ga-PSMA. HPLC was equipped with a Hitachi L-7100 pump coupled with ultraviolet (Hitachi L-7400) and radiometric (γ-Raytest-Isotopenmessgeräte GmbH) detectors. Solvents for HPLC were obtained as HPLC-grade and degassed by ultrasonication for 15–20 min before use. The gradient elution system used mobile phase A (deionized H2O + 0.1% trifluoroacetic acid) and mobile phase B (acetonitrile) with a flow of 1 mL/min.
Manual 68Ga Labeling
68Ga was eluted with 5 mL of 0.1 M HCl and subsequently postprocessed online according to a previously published procedure (15). For labeling with ethanol-based 68Ga eluate (N5: 90% ethanol/0.9N HCl), 0.10–0.70 μg (0.11–0.74 nmol) of PSMAHBED was added to a mixture of buffer and 0.1–1 mL of 68Ga eluate. The influence of buffer (molarity, volume, pH), amount of ligand, volume of eluate, temperature, and reaction time was investigated.
For clinical application, 0.75–5 μg (0.79–5.28 nmol) of PSMAHBED were added to a mixture of 1,000 μL of 1 M ammonium acetate buffer and 1 mL of ethanol-based 68Ga eluate (1.85-GBq 68Ge/68Ga generator; iThemba Labs). The mixture, with a final pH of 3.9–4.2, was heated for 5 min at 85°C in a closed 10-mL vial followed by sterile filtration and dilution with 10 mL of saline solution.
Synthesis without postprocessing was performed as follows: 1 μg (0.11 nmol) of PSMAHBED was added to a mixture of 600 μL of 3 M ammonium acetate buffer and 2 mL of 68Ga in 0.6N HCl (1.85-GBq 68Ge/68Ga generator; iThemba Labs). The mixture, with a final pH of 4.2, was incubated for 5 min at 40°C in a closed 10-mL vial.
Automated Tracer Synthesis
68Ga obtained from a 1.1-GBq 68Ge/68Ga generator (IGG100; Eckert & Ziegler Strahlen- und Medizintechnik AG) with a TiO2 matrix was eluted with 0.1N HCl and postprocessed with ethanol/HCl solution according to a method described in the literature (13,15). PSMAHBED was labeled by adding aliquots (5, 10, 15 μL = 5, 10, 15 μg = 5.28, 10.56, 15.84 nmol) of a PSMAHBED stock solution (1 mg/mL) to mixtures of postprocessed 68Ga eluate (800 μL) and 1 M NaOAc solution (1.6 mL, pH 7), which corresponds to an ethanol content of 33 vol% of the crude reaction solution using the small radiolabeling synthesizer Modular-Lab eazy (Eckert & Ziegler Strahlen- und Medizintechnik AG) and a temperature of 110°C. Radiochemical yields were determined after a 200- and 300-s reaction time.
Quality Control
TLC was performed with 1-μL aliquots on TLC or ITLC silica gel plates after labeling for 1, 3, 5, and 10 min and subsequently developed in different solvent systems. Analyses were performed using a flat-bed scanner (Instant Imager [Packard Canberra] or Rita Star [Raytest Isotopenmessgeräte GmbH]).
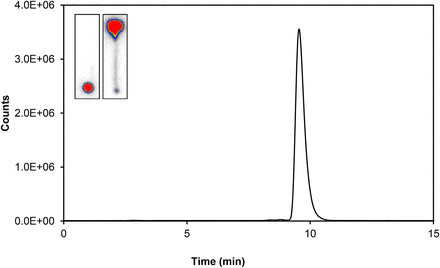
The results were compared with radio-HPLC, which was performed using 2 gradient systems depending on labeling method. The gradient elution system used mobile phase A (deionized H2O containing 0.1% trifluoroacetic acid) and mobile phase B (100% acetonitrile) and a low rate of 1.0 mL/min. Starting with 100% A/0% B, the gradient was increased to 100% B over 15 min and then returned to the initial gradient conditions within 5 min. The retention time of free 68Ga was 2.8 min; 68Ga-PSMAHBED eluted at 9.5 min.
RESULTS
68Ga Labeling
Currently, fractionated 68Ga eluate is regularly used for radiosynthesis of 68Ga-PSMAHBED. The disadvantage of fractionation is the content of metallic impurities such as 68Ge generator breakthrough and stable 68Zn generated from 68Ga decay (16), which are decreased but, in fact, not chemically removed in this case. It is therefore desirable to find optimized conditions using postprocessed 68Ga for 68Ga-PSMAHBED labeling, with the postprocessed 68Ga fraction meeting recommendations for 68Ge/68Ga radionuclide generator eluates as described in the monograph “Gallium (68Ga) Chloride Solution for Radiolabeling” of the European Pharmacopoeia (17).
The 68Ga eluate contains measurable activities of the long-lived 68Ge, which is a critical parameter in the context of the routine clinical application of 68Ga-radiopharmaceuticals (18,19). Breakthrough in commercial 68Ge/68Ga generators varies over time and by frequency of use. Typical values of initial 68Ge breakthrough (68Ge present in the eluate divided by 68Ga present in the eluate) are on the order of 0.0001%–0.00001%. Over time, this ratio increases because of the decreasing amount of generated and eluted 68Ga. According to the certificates for each individual generator, in particular the GalliaPharm (Eckert & Ziegler Strahlen- und Medizintechnik), there is a guarantee that both initial and permanent 68Ge breakthrough will be less than 0.001%, which is recommended by the European Pharmacopoeia for the synthesis of 68Ga radiopharmaceuticals (17). For 68Ge/68Ga generators with higher levels of 68Ge breakthrough, online or offline purification to remove 68Ge from the initial 68Ga eluate is vital. In addition to 68Ge breakthrough, the relatively large volume, high acidity of the eluate, and presence of further metal ion contaminants such as Zn(II) and Fe(III) are problems addressed by these so-called postprocessing procedures. There are no defined limitations to metal contaminants, but research has shown that trivalent metal cations, in particular, can hinder efficient radiolabeling with 68Ga. In addition, a reduction in the labeling yield and in specific activity occur inasmuch as metal contaminants compete with the low amounts of 68Ga (1 GBq 68Ga = 9.731 pmol = 6.61 pg) available for complex formation with the precursor.
Several methods have been developed to reduce metallic impurities and concentrate the eluate, of which variations of cation-exchange resin–based postprocessing have been particularly successful (13,20). The initial method pioneered by Zhernosekov et al. using acetone/hydrochloric acid solutions provides high recovery of 68Ga and complete removal of 68Ge, as well as a decrease in acidity, volume, and other metallic impurities (13). A suitable and efficient variation is cation-exchange–based postprocessing using ethanol/hydrochloric acid medium (15). It equally allows concentration of 68Ga generator eluate, removal of metal impurities, and quantitative removal of 68Ge breakthrough, ensuring that the final injectable radiopharmaceutical fulfills regulatory requirements relating to 68Ge content. A recently published study confirmed the hypothesis that ethanol facilitates incorporation of the radiometal (21). The radiolytic protection capability of ethanol additionally benefits a labeling reaction being performed with high activities.
Using a modified labeling method published by Eder et al., optimization was conducted as part of this study (1), resulting in the finding that 0.25 M (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) buffer (pH 7.5) is the most suitable system for radiolabeling of PSMAHBED at a very low precursor concentration using 1 mL of postprocessed eluate. Using these conditions, labeling yields were found to be noticeably dependent on reaction temperature. Although HBED is a nonmacrocyclic chelate, 68Ga-complex formation yields are relatively low at ambient temperature, that is, between 25°C and 40°C, and do not exceed 40% after 10 min of reaction time. In contrast, yields and complex formation kinetics are high and fast at elevated temperatures of 60°C and 90°C. At a 10-min reaction time, labeling yields were equivalent for both temperatures (Fig. 1).
Radiolabeling yields with various reaction temperatures using ethanol-postprocessed 68Ga-eluate (0.1 μg/0.11 nmol/0.025 μM PSMAHBED; 1 mL of N5; 3 mL of 0.25 M HEPES, pH 7.5; overall reaction volume, 4 mL; n = 3).
Using elevated temperatures and increasing the amount of precursor to 0.7 μg (0.74 nmol), complex formation occurs quickly and reliably. Radiolabeling yields of more than 90% are achievable within 1 min and of more than 95% within 3 min (Fig. 2). These conditions fulfil regulatory requirements without the need for further purification when using a reaction time of 5 min.
Radiolabeling yields with various precursor amounts (90°C; 1 mL of N5, 3 mL of 0.25 M HEPES, pH 7.5; overall reaction volume, 4 mL; n = 3).
In the context of clinical applications, HEPES is not necessarily the buffer of choice, although it is not biologically critical and offers high incorporation of radioactivity and, accordingly, high specific activities. Because there is no monograph of PSMAHBED listed in the pharmacopeia, the radiopharmaceutical has to be purified of HEPES and an additional quality control step is necessary to determine the residual in the final formulation (as described as part of the monograph for 68Ga-DOTATOC). To circumvent additional purification steps, 1 M NH4OAc solution was used as a buffer medium as part of this study.
Using 900 μL of 1 M NH4OAc mixed with 1 mL of postprocessed 68Ga-eluate resulted in a labeling pH of 3.9–4.2. A labeling temperature of 85°C was found to be optimal for clinical routine production using ethanol-postprocessed 68Ga eluate. Figure 3 shows radiolabeling kinetics depending on precursor amount using routine production conditions. When using higher activities (>1 GBq) for labeling, more precursor was necessary to obtain satisfactory and reproducible radiochemical yields. Radiolabeling with less than 1 μg (1.1 nmol/0.526 μM) PSMAHED suffers from low reproducibility (±10.3%) and low yields. The use of more than 1 μg (1.1 nmol/0.526 μM) PSMAHBED leads to radiolabeling yields of more than 98% within 5 min of reaction time. In this case, additional purification of the product can be omitted as it already fulfils regulatory requirements. As a variation, radiolabeling of 1 μg (1.1 nmol/0.423 μM) PSMAHBED was performed using fractionated 68Ga eluate at elevated temperature (40°C). In this case, radiolabeling yields of 75.0% ± 5.8% were obtained requiring additional purification of the product before injection. When the results of fractionated and ethanol-based postprocessed 68Ga are compared, the latter are superior.
Radiolabeling yields with various precursor amounts using postprocessed 68Ga-eluate (85°C; 1 mL of N5; 900 μL of 1 M NH4OAc, pH 3.9–4.2; n = 3).
Transferring the investigated radiolabeling method without further changes to an automated module system (Modular-Lab eazy) was easily achieved. Taking the different heat transmission rate of the reactor into account, higher temperatures than for manual synthesis are necessary. Without further optimization of the conditions toward automatization, radiolabeling yields of 93% ± 3.2% were obtained within 200 s (3.3 min) using the minimum amount of precursor (5 μg/5.28 nmol) recommended by Eckert & Ziegler. An extension of reaction time up to 300 s (5 min) did not affect yields (91% ± 4.5%).
TLC Analytics
So far, quality control of 68Ga-PSMAHBED has been performed by means of radio-HPLC (1). Keeping in mind that the time needed for quality control of short-lived nuclides should not exceed the time needed for synthesis, obtaining higher product activities by shortening the time for quality control is crucial when developing novel procedures for routine clinical application. For example, performing a 20-min HPLC protocol (as suggested by Eder et al. (1)) would reduce the absolute 68Ga-PSMAHBED product radioactivity by 18%. In this case, the use of TLC/ITLC appears to be an attractive alternative because the method is generally expected to allow faster but still reliable quality control with little equipment complexity and accordingly cost. Thus, a TLC/ITLC system is required to differentiate between 68Ga and 68Ga-PSMAHBED, making use of the advantages of this quality control method. To find optimum conditions for TLC/ITLC quality control of 68Ga-PSMAHBED, different mobile phases on silica gel 60 and on ITLC silica gel plates were investigated. General separation ability was evaluated with silica gel 60 plates as the stationary phase and several mobile phases. The focus was set on duration of development and separation ability of the investigated TLC systems. The documented Rf values are summarized in Table 1.
Rf Values for Investigated Mobile Phases Using Silica Gel 60 or ITLC Silica Gel Plates as Stationary Phase
With the exception of acetonitrile (6) and cyclohexanone (7) mixtures, all investigated mobile phases are suitable for separating 68Ga from 68Ga-PSMAHBED on silica gel 60 plates. Comparison with radio-HPLC results confirmed the high reliability of mobile phases 1–3. Altogether, 3 mobile phases were found to be suitable for TLC analytics of 68Ga-PSMAHBED using silica gel 60 plates.
Even though TLC is a reliable low-budget method, the development of the plates takes too long to have an advantage over the established 20-min HPLC procedure. In a second step, ITLC silica gel plates were investigated using mobile phases 1–3 and 5 to shorten the development time of plates in the solvent chamber. All observed Rf values and development times using ITLC silica gel plates as the stationary phase are summarized in Table 1.
Figure 4 shows radio-TLC (left image) and ITLC (right image) images developed in mobile phases 1–3 and 5, with free 68Ga (left lane) directly compared with 68Ga-PSMAHBED (right lane). As anticipated, separation depends on both the mobile phase and the stationary phase because of changes in the interaction dependent on component polarity. As a result, not all investigated mobile phases are suitable for development of both TLC and ITLC, as shown in Table 1 and Figure 4. Altogether, it was possible to find 2 mobile phases (phases 2 and 3) that can be used with silica gel 60 (TLC) and ITLC silica gel plates to determine the radiochemical yield of 68Ga-PSMAHBED for quality control. Quality control was completed in less than 10 min using mobile phases 2 and 3 with ITLC silica gel plates. Compared with more than 15 min for quality control by means of radio-HPLC, this is a fast and easy-to-handle low-budget method with high reliability.
Images of radio-TLC (left plate) and ITLC (right plate) developed in mobile phases 1–3 and 5. M = 68Ga; L = 68Ga-PSMAHBED; 1 = 0.1 M Na3C6H5O7, pH 4; 2 = 1:1 MeOH/NH4OAc; 3 = 3:1:1 5% NaCl/MeOH/25% NH3; 5 = 9:1:0.5 MeOH/0.9% NaCl/1 (mg/mL) EDTA.
All analytic data obtained by TLC and ITLC silica gel were also verified by means of HPLC (Fig. 5).
Radio-HPLC of 68Ga-PSMAHBED for verification of TLC (left lane, 0.1 M Na3C6H5O7, pH 4) and ITLC (right lane, 1:1 MeOH/NH4OAc) quality control.
DISCUSSION
68Ga-PSMAHBED is a promising new 68Ga-PET tracer that is being increasingly applied for diagnosis of various diseases related to primary prostate cancer and other cancers, such as renal cell carcinoma, that also express PSMA in the neovasculature (22). A process of replacing previously used tracers, such as 18F-choline, with 68Ga-PSMAHBED has already begun on the basis of the promising results continually being published. Compared with previous 18F-based PET tracers, the synthesis of 68Ga-PSMAHBED exemplifies the advantages of radiometal-based PET tracers. One may soon expect the availability of kit-analog preparations, as recently reported for a 68Ga-octreotide derivative (23).
However, those syntheses should be robust and reliably guarantee radiochemical labeling yields higher than 99%, making subsequent purification steps unnecessary. In the case of 68Ga-radiopharmaceuticals, an additional isolation of 68Ge via postprocessing procedures or quality control for 68Ge breakthrough in the product synthesized should become redundant.
The present study was able to modify the synthesis of 68Ga-PSMAHBED by adopting established 68Ge/68Ga generator postprocessing methods to eliminate 68Ge breakthrough before 68Ga-labeling. Because acetone- and ethanol-driven cation-exchange postprocessing pathways are online, fast, and almost quantitative, the yield of 68Ga-PSMAHBED labeling is not affected. Labeling yields of more than 99% are achieved at optimized conditions, and product becomes available within 5 min after generator elution—including postprocessing. The synthesis is transferable to automated modules such as the Modular-Lab eazy, achieving acceptable yields even at a lower pH. It was possible to develop fast and reliable TLC- and ITLC-based methods that provide results comparable to the established HPLC method. This is important in clinical applications for which rapid and stable quality control is indispensable. Because the gain in product activity due to the short synthesis period will decrease whenever longer periods are required for quality control (such as a 20-min HPLC protocol as suggested by Eder et al. (1)), the new ITLC quality control is of special importance and can be terminated within 5 min using a fast, reliable, low-cost radio-ITLC method with little equipment complexity. Analytic data obtained with this ITLC system are confirmed by HPLC.
CONCLUSION
Adaptation of the initially described synthesis of the 68Ga tracer (for nonpostprocessed 68Ge/68Ga generator eluates) to state-of-the-art procedures for cation-exchange–based eluate purifications has been straightforward. Radiolabeling yields are nearly quantitative. The synthesis is completed within 5 min, providing labeling yields of more than 95% and specific activities of more than 326 ± 20 MBq/nmol, making subsequent product purification obsolete. The synthesis, as well as the TLC/ITLC quality control methods, was successfully implemented in the systematic clinical protocols for over 200 patient studies.
DISCLOSURE
Eckert & Ziegler Eurotope GmbH (Berlin, Germany) provided the automated system Modular-Lab eazy. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jan. 12, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication May 9, 2016.
- Accepted for publication October 2, 2016.