This special supplement to The Journal of Nuclear Medicine is a detailed tabulation of literature on FDG PET in oncology (1993–June 2000), cardiology (1986–June 2000), and neurology (1980–June 2000). This document is a subset of the original document formally submitted to the Health Care Financing Administration (HCFA) in July 2000 to request expanded Medicare reimbursement for FDG PET. It has been improved by eliminating any errors in tabulation and further clarified as the result of comments from an independent review of the original HCFA submission. This document also differs from the original HCFA submission in that it does not include some background sections and lacks references that were identified but not used because of specific inclusion and exclusion criteria.
The goal of this document is to provide a summary of all FDG PET literature for the specified periods, with tabulated values of sensitivity, specificity, percentage in management changes, etc. This document is not intended to be a formal meta-analysis or cost-effectiveness analysis of the available literature. Instead, it is meant to provide an overview of the available literature, so that future detailed studies can use this document as a starting point.
Because of the difficulty of searching all FDG PET literature, this document inevitably does not include some research articles and abstracts that may be useful. The authors have tried to make the search as comprehensive as possible, but some literature may have been overlooked. Details of the literature search strategy are provided in Appendix A. Although a formal meta-analysis is not performed, a simple weighted averaging of data using various strategies is presented (Appendix B). This weighted averaging is meant to give only a general indication of the overall accuracy values and, therefore, should be interpreted with care. A data pooling analysis is also included.
The document is organized to show, first, how the tabulated data should be interpreted. This is followed by oncologic, cardiac, and neurologic application sections that provide, for each disease process, a disease background section, a case example illustrating the clinical implementation of FDG PET, an explanation of why FDG PET helped, a key management issues section (see also Maisey et al. (285)), and a summary of evidence for FDG PET with management change data for the disease and references to the relevant full literature search (in tabulated form) for the accuracy of FDG PET in specific applications. The numbers of patient studies utilized in calculating summary management changes are displayed in Tables 20 and 24 along with management figures and are embedded (without display) within the individual spreadsheets as selected from the data lines that report management change information. In addition, a summary of results from the literature search on FDG PET in all cancers is provided, as well as a summary of FDG PET literature searched for the oncologic, cardiac, and neurologic applications. A full reference list is also provided at the end. Appendix A gives details on the way in which literature was searched and analyzed, and Appendix B reports some alternate approaches to summarizing the data.
The average FDG PET sensitivity and specificity across all oncology applications are estimated at 84% (based on 18,402 patient studies) and 88% (based on 14,264 patient studies), respectively. The average management change across all applications is estimated to be 30% (based on 5,062 patients). These data were obtained combining 419 total articles and abstracts on studies in which FDG PET was used. Various methods of analysis were applied to these data (Table 25), which revealed only a small amount of variation in the ratio values. Specifically, the sensitivity of PET ranged from 84%–87%, the specificity ranged from 88%–93%, and the accuracy ranged from 87%–90%.
At the time of submission of this work, HCFA had just announced expanded coverage for FDG PET to include imaging for various aspects of lung, colorectal, esophageal, and head and neck cancers and melanoma and lymphoma. In addition, coverage for seizure work-ups and myocardial viability was approved. We are confident that with continued acquisition of data from well-designed clinical studies, true broad coverage for FDG PET can soon be a reality. We hope that readers of the journal will find this to be a valuable resource in better understanding the existing diversity of literature available for FDG PET.
INTERPRETING SPREADSHEETS IN THIS DOCUMENT
This report contains spreadsheets summarizing all FDG PET literature. A spreadsheet is provided for each disease under consideration, along with summary spreadsheets (see Table 1, lung cancer, as an example). On each specific disease spreadsheet, the name of the disease appears in the upper left-hand corner. The data is broken down into applications of FDG PET for diagnosis, staging, diagnosis and staging, recurrence, monitoring response, and other applications. Some diseases include a mixture of these applications and, therefore, have multiple listings in several categories.
For each disease, the first author and year of publication of the article or abstract are listed in the far left column. The second column designates “A” for abstract or “RA” for research article. The third column lists the purpose of the study. The fourth column lists the total number of patients who were included initially in the study. The fifth column lists the total number of patient studies actually implemented and upon which results data were calculated (sometimes less than the total in the fourth column, because of patient drop out or other causes, and sometimes greater than the total in the fourth column, because multiple PET scans may have been counted). In some applications in which lesions were counted, a column is also listed for the number of lesions studied. Studies using nondedicated PET are indicated. Several additional columns show the percentages for sensitivity, specificity, predictive value, and accuracy for FDG PET and CT. The gold standard used for verifying FDG PET results is also listed in a separate column. If percent management changes were available, they are listed in the last column. Finally, beneath each table are footnotes highlighting details from specific studies to further clarify how the study populations were either composed or counted. Abbreviations used throughout the tables are listed alphabetically in Appendix C.
A summary for each application is provided in bold. The bolded summary totals for number of patients, number of patient studies, and number of lesions reflect the totals retrieved from the literature for all studies listing data that is inclusive of that application. Each study entry is listed with complete reported data that may include data relevant to several applications. This accounts for data repeated across applications within a given spreadsheet and is discussed in Appendix A. From within each application are selected the respective study data applicable to that application (e.g., recurrence data in instances in which both staging and recurrence data may have been listed in a given entry) and used in the weighted average formulas generating that application’s summary values, which are listed in the statistical ratio columns. These selected N values for total patient studies and total lesions do not appear in the individual spreadsheets but are embedded in the formulas and appear with the application’s summary values in the overall summary sheets at the end of this report. Note that a weighted average is used, which weights studies by the number of patients, so that results obtained with more patients are given more credit. If lesion-by-lesion analysis was performed, a separate value for that analysis is also listed.
All tabular matter is presented here in the form in which it was submitted to HCFA, with the exception of various corrections to tabulation errors found in certain spreadsheets and their carryover to the overall summary sheets, and the placement of table numbers according to the style of this journal.
ONCOLOGIC APPLICATIONS
Lung Cancer
Disease Background.
Lung cancer is among the most frequent and most lethal of cancers striking both men and women. It is the most rapidly increasing tumor in industrialized countries. Most lung cancers are caused by smoking. However, smoking is a less important factor in adenocarcinoma, the lung cancer most rapidly increasing in the United States. Lung cancer accounts for 22% of all cancers in men and 8% of all cancers in women. Five-year survival is achieved by only 13% of all lung cancer patients. Basic treatment for non-small cell lung cancer (NSCLC) is surgical, with only 20% of patients presenting as operable. Patients who are not operable receive palliative chemotherapy or radiation. Small cell lung cancer patients respond well initially to chemotherapy and radiation and generally do not undergo surgery. Their long-term prognosis is poor.
Case Example.
A 62-y-old patient with known NSCLC was evaluated before planned lobectomy. The patient had no symptoms (e.g., headaches). FDG PET revealed extensive metastatic disease to the brain in addition to the primary cancer in the lung.
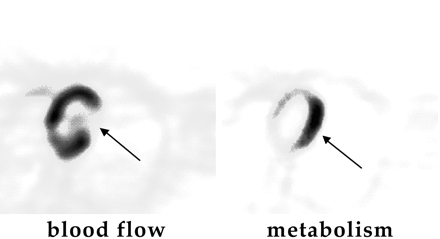
Why Did FDG PET Help? Because the FDG PET scan showed that the patient had much more extensive disease than previously thought (Fig. 1, arrows), lobectomy was not a management option for this patient. The patient had no symptoms related to the brain metastases, but the FDG PET whole-body survey scan caught tumor spread to the brain.
Key Management Issues.
-
Diagnosing the lung mass
-
Staging NSCLC
-
Assessing recurrence
-
Monitoring response to therapy
Summary of Evidence for FDG PET in Lung Cancer.
For staging: An estimated 37% change (Table 1) was noted in management effect, based on 1,565 patient studies (Table 24).
Colorectal Cancer

Disease Background.
The colon and the rectum are parts of the large intestine and are responsible for absorption of various substances not absorbed by the small intestine. In western industrialized countries, colorectal cancer is the second most common cause of death from cancer. However, 20-fold variations in international incidence rates have been noted, with the highest rates found in Connecticut in the United States. Primary treatment is surgical, leading to a 50% 5-y survival rate. Adjuvant chemotherapy (chemotherapy before removal of the tumor) is now more commonly used. Radiation is sometimes used for rectal carcinoma and less often for colon cancer. Approximately 20% of patients with recurring cancers are eligible for further resection, with half relapsing early because of previously unidentified metastatic sites. Imaging helps to determine the spread (or lack thereof) of the primary tumor in the colon or rectum.
Case Example.
A patient with carcinoma of the rectum was treated with surgery and radiotherapy. One year later, results of a blood test indicated rising carcinoembryonic antigen (CEA) levels. A CT scan did not reveal the site of tumor recurrence. An FDG PET study showed a liver focus (Fig. 2, arrows), which was proven by biopsy to be recurrent rectal cancer.
Why Did FDG PET Help?
The liver metastasis was identified as the likely source of this patient’s rising CEA blood marker. No other source was apparent. The patient, therefore, could be managed with this information. For patients with isolated liver recurrence, surgery for removal of a part of the liver is usually a good option.
Key Management Issues.
-
Evaluating suspected recurrence and restaging
-
Assessing response to treatment
-
Evaluating liver lesions for metastatic disease
Melanoma

Disease Background.
With an increasing mortality rate second only to that of lung cancer, malignant melanoma is the most rapidly increasing cancer in white populations, with incidence increasing at >5%/y since 1973. The most common cancer striking young women between ages 25 and 29, melanoma accounts for 18% of all cancers in young adults 15–39 y old. Melanoma risk factors include preexisting skin lesions and lighter hair color, with red-haired and blond individuals having 3 and 2 times greater risk than average, respectively. An overall increase in risk appears related to strong solar ultraviolet radiation. Approximately 20% of patients who present with nodal metastases with no distant metastases are cured by surgery. For isolated metastases to the brain and lung, surgery can improve survival. Thus FDG PET’s role in identifying truly isolated metastases is central to the making of rational decisions about radical surgical removal of metastases.
Case Example.
A 63-y-old patient had a melanoma removed from the skin overlying the right scapula (shoulder region). A second metastasis was excised at the nape of the neck. An FDG PET scan was ordered to stage the patient’s cancer. Increased FDG metabolism was seen after surgery at the shoulder site (Fig. 3, far right, top arrow). In addition, multiple metastases were seen within the anterior mediastinum, left lung, left adrenal, left axilla, and para-aortic nodes.
Why Did FDG PET Help?
The FDG PET scan showed that the melanoma had spread to various tissues and that chemotherapy would be the only option.
Key Management Issues.
-
Determining the stage of thick melanoma lesions at presentation
-
Assessing nodal spread from lesions of intermediate thickness
-
Confirming the recurrence of disease
-
Monitoring response to treatment
-
Restaging before surgical removal of isolated metastases
Summary of Evidence for FDG PET in Melanoma.
For staging: An estimated 26% change was noted in management effect, based on 283 patients (Table 3).
Lymphoma

Disease Background.
Both Hodgkin’s disease (HD) and non-Hodgkin’s lymphoma (NHL) are common malignancies that are increasing in frequency. The underlying problem in lymphoma arises from the individual’s white blood cells, cells involved in helping to fight infections. Significant differences exist between HD and NHL, and these differences factor into both diagnosis and treatment. HD begins as a unifocal disease located in a single group of malignant lymph nodes and spreads via adjacent associated lymph node groups. Limited disease is treated appropriately with radiation therapy, resulting in complete cures for a high percentage of patients. Even after recurrence, treatment still may result in permanent cure. Patients with advanced disease have a poorer prognosis and usually require chemotherapy in addition to other treatments. NHL is a multifocal disseminated disease, usually requiring combined chemotherapy, sometimes radiotherapy, and, in some instances, high-dose chemotherapy with bone marrow transplantation. In most patients the disease is ultimately fatal. However, long remission and cure can be induced effectively in high-grade tumors that would be rapidly fatal if untreated. Low-grade NHL, which has a better prognosis untreated, does not respond as well to chemotherapy and consequently can result in a worse prognosis after treatment.
Case Example.
A 27-y-old man with lymphoma underwent an FDG PET study before chemotherapy in July 1999 (Fig. 4, top row). At that time, evidence of cancer was found in the right shoulder and thoracic spine (arrows). The first follow-up FDG PET scan (Fig. 4, middle row) showed nonspecific bone marrow response to chemotherapy (a common finding). The second follow-up scan (Fig. 4, bottom row) demonstrated complete remission, with the right shoulder and thoracic spine regions no longer showing increased FDG metabolism.
Why Did FDG PET Help?
FDG PET showed that the chemotherapy was working and that the cancer cells were being destroyed. This helped doctors know that further treatment was not needed at that time and gave the patient a sense of relief that his condition was improving.
Key Management Issues.
-
Staging the disease before treatment
-
Monitoring response to treatment
-
Detecting recurrence
-
Making a differential diagnosis
Summary of Evidence for FDG PET in Lymphoma.
For staging: An estimated 21% change was noted in management effect, based on 407 patient studies (Table 4).
For diagnosis/staging: An estimated 5% change was noted in management effect, based on 62 patient studies (Table 4).
For recurrence: An estimated 10% change was noted in management effect, based on 158 patient studies (Table 4).
Head and Neck Cancer

Disease Background.
Cancer of the head and neck is relatively uncommon in the western world, occurring in 2%–4% of all cancers. In contrast, it comprises up to 40% of all cancers in some Asian countries. In the western cases, the majority are squamous cell tumors with a variable aggressiveness that depends on site and histological appearance. Strong environmental links have been found with tobacco and alcohol usage and with other factors, such as chemicals, fumes, and viruses. Multidisciplinary teams of head and neck surgical oncologists, radiation oncologists, imaging specialists, and medical oncologists operating in specialized centers are required for good outcomes. Treatment is directed at maintaining the form and function of the head and neck structures as well as eradicating the disease. Because of the need to limit surgery and the fact that local nodal spread is the most important prognostic factor, imaging has an important role in the management of these tumors. After treatment, conventional anatomical imaging procedures prove less useful because of the distortion of anatomy caused by treatment. Therefore, FDG PET is of particular importance in follow-up imaging of suspected recurrence.
Case Example.
A patient with a right alveolar ridge carcinoma was referred for FDG PET scanning before surgery for staging purposes. Results of a CT scan (Fig. 5A, 5C) indicated that the tumor extended superiorly into the maxillary sinus. Registered PET and CT images showed uptake of FDG within the primary site arising from the alveolar ridge (Fig. 5B) but no evidence of tumor within the sinus itself (Fig. 5D). This illustrates how the FDG PET scan can identify the extent of disease when inflammatory tissue and tumor are co-located.
Why Did FDG PET Help?
FDG PET helped because it determined that the tumor was localized and did not extend into the maxillary sinus. This directly aided the surgery for tumor removal.
Key Management Issues.
-
Locating the site of primary disease
-
Determining the extent of primary disease
-
Staging of lymph node spread
-
Detecting recurrence
-
Assessing response to therapy
Summary of Evidence for FDG PET in Head and Neck Cancer.
For diagnosis/staging: An estimated 33% change was noted in management effect, based on 15 patient studies (Table 5).
For recurrence: An estimated 33% change was noted in management effect, based on 15 patient studies (Table 5). Because management effect for both diagnosis/staging and recurrence is based upon the same single study of 15 patients, results should be interpreted with caution.
Breast Cancer

Disease Background.
In the United States, breast cancer is currently second only to lung cancer as the leading cancer causing death in women. It is the most common single cause of death for women ages 35–50 y. Cure can be achieved with early diagnosis and treatment, but a multidisciplinary approach is required. Treatment includes surgery, which is becoming progressively less radical, together with chemotherapy. Hormone and radiation therapy also are used ther-apeutically. Imaging is an important part of detection, staging, and management of most breast cancer patients. Although mammography has helped to detect breast cancer in many women, many cancers are missed in women who have dense breasts, implants, or have been treated previously for breast cancer. In addition, more methods are needed to better detect the spread of breast cancer and to monitor treatment and recurrence.
Case Example.
A 61-y-old woman with breast cancer showed several foci of tumor involvement in the chest and spine (Fig. 6, top row) on her initial FDG PET scan. After chemotherapy, an FDG PET study was requested to look for tumor response to chemotherapy (Fig. 6, bottom row). The small foci of FDG accumulation seen throughout the chest and spine clearly had resolved. Post-therapy CT was positive (still showed tumor mass) because of necrosis and edema from therapy.
Why Did FDG PET Help?
FDG PET showed that the chemotherapy was working and that this patient’s breast cancer had responded to this particular type of chemotherapy. These changes were evident long before the CT scan showed any signs of response to treatment.
Key Management Issues.
-
Determining if a breast mass is benign or malignant (This is especially difficult in dense breasts, implants, and after treatment. Approximately 60%–85% of breast biopsies are benign.)
-
Staging of axillary and internal mammary lymph nodes
-
Detecting metastatic disease
-
Detecting local or distant recurrence
-
Assessing the response of the tumor to treatment
Summary of Evidence for FDG PET in Breast Cancer.
For diagnosis: An estimated 100% change was noted in management effect, based on six patient studies (Table 6). Because of the limited number of patient studies upon which this management change is based, this value should be interpreted with caution.
For staging: An estimated 24% change in management effect, based on 111 patient studies (Table 6).
For recurrence: An estimated 40% change in management effect, based on 23 patient studies (Table 6).
Brain Tumors

Disease Background.
The incidence of primary brain tumors in the population is 11 in 100,000, with overall metastatic brain disease being more common. Typically, space-occupying lesions are caused by primary tumors, with >50% of patients presenting with some form of epilepsy. New treatments are being introduced, including guided biopsy and surgery (which are frequently image guided), targeted radiation, chemotherapy, and radioactive seed implantation. Outlook remains poor, with survival <1 y for patients with high-grade tumors. Imaging is increasingly required to detect disease, particularly recurrent disease, and in planning and guiding therapy and biopsy. An especially difficult task is determining if cancer has come back after radiation therapy.
Case Example.
The preferred treatment for brain tumors is surgical removal. FDG PET scans are useful for evaluating the efficacy of surgical procedures. A 64-y-old woman with a diagnosis of glioblastoma multiforme (aggressive brain tumor) was operated on to remove the tumor and was treated with radiation. Subsequent contrast-enhanced MRI (Fig. 7, left) suggested possible tumor recurrence. Note the area of contrast accumulation near the surgical region (white arrow). The lack of a corresponding FDG accumulation in that region in the FDG PET image (Fig. 7, right) suggested that the contrast enhancement observed in the MR image was the result of radiation necrosis and that no residual tumor was present at that time.
Why Did FDG PET Help?
FDG PET helped by showing that an inconclusive finding on MRI was, in fact, the result of radiation and not residual tumor. Therefore, this patient did not need medical or surgical intervention.
Key Management Issues.
Initial management.
-
Diagnosing and grading the malignancy
-
Determining the extent for treatment planning
-
Directing biopsy
-
Determining prognosis
Post-treatment management.
-
Differential diagnosis between recurrence and radiation necrosis
-
Directing biopsy (This helps to determine where in the brain to sample the tissue, by differentiating tumor from necrosis and edema.)
-
Determining the extent of tumor in treatment planning
-
Monitoring response to treatment (surgery/radiotherapy/chemotherapy) (This involves differentiating tumor from necrosis and edema to determine how well the treatment affected the tumor.)
Summary of Evidence for FDG PET in Brain Tumors.
For recurrence: An estimated 31% change was noted in management effect, based on 89 patient studies (Table 7).
Ovarian, Cervical, and Uterine Cancer

Disease Background.
Ovarian cancer is the fifth leading cause of cancer death in women in the United States, with 14,500 deaths and 25,400 new cases diagnosed each year. Approximately one-third of all new cases will have metastatic disease at the time of diagnosis, with another third developing clinical metastases during the first year after surgical resection. The current recommendation for management of patients without evidence of metastatic disease at 1 y after diagnosis is to perform second-look laparotomy for clinical staging and possible tumor resection. For early-stage ovarian cancer, accurate diagnosis is very difficult.
Cervical cancer is one of the most common cancers, accounting for 6% of all malignancies in women, with an estimated 16,000 new cases of invasive cancer of the cervix and 5,000 deaths in the United States each year. The prognosis for this disease is markedly affected by the extent of disease at the time of diagnosis.
Cancer of the endometrium, a common type of cancer in women, is a disease in which cancer cells are found in the lining of the uterus (endometrium). Cancer of the endometrium is different from cancer of the muscle of the uterus (sarcoma of the uterus). Cancer of the endometrium is the most common pelvic gynecologic malignancy and accounts for 13% of all cancers in women. It is a highly curable tumor.
Case Example.
A 50-y-old woman with a history of ovarian cancer showed rising tumor markers in an annual blood test that looked for possible tumor recurrence. A follow-up CT scan was unable to find the source of the recurrence. An FDG PET study showed that the tumor had metastasized to the right lobe of the liver (Fig. 8, arrows on site of metastasis viewed on 4 different sections through the whole body). No other areas of metastasis were seen.
Why Did FDG PET Help?
FDG PET showed that the blood study was correct (it was not falsely elevated) and that the source of recurrence was the liver. This patient was confirmed through follow-up to have recurrence in the liver.
Key Management Issues.
-
Staging lymph nodes
-
Identifying recurrent disease after surgery and radiation
-
Assessing response to treatment
Summary of Evidence for FDG PET in Ovarian, Uterine, and Cervical Cancer.
For recurrence: An estimated 17% change was noted in management effect, based on 30 patient studies (Table 8).
Bladder Cancer

Disease Background.
Bladder cancer is a disease in which cancer cells originate from the bladder wall. Approximately 70%–80% of patients with newly diagnosed bladder cancer will present with superficial bladder tumors. Those tumors that are noninvasive are often curable, and those that are deeply invasive are sometimes cured by surgery, irradiation, or a combination of modalities that includes chemotherapy. Some patients with distant metastases have achieved long-term complete response after treatment with combination chemotherapy regimens. The major prognostic factors in carcinoma of the bladder are the depth of invasion into the bladder wall and the degree of differentiation of the tumor. Transurethral surgery, intravesical medications, and cystectomy (bladder removal) have been used in the management of patients with superficial tumors and are all associated with 5-y survival rates for 55%–80% of patients treated. As with many cancers, the key to management is determining if the bladder cancer has spread beyond the bladder to the local lymph nodes or to distant parts of the body.
Case Example.
A patient with cancer of the bladder was scanned for staging purposes. Focal increased FDG uptake was seen within the posterior aspect (back) of the bladder, indicating primary disease only (Fig. 9, arrow). Mild accumulation of FDG also was seen around a right total hip replacement (Fig. 9A), possibly indicating active inflammation or infection, although the patient did not complain of any hip pain.
Why did FDG PET help?
FDG PET helped by showing no evidence of cancer spread beyond the bladder, so that local treatment (e.g., removal of the bladder) likely would benefit the patient.
Key Management Issues.
-
Primary nodal staging
-
Systemic metastases staging
Summary of Evidence for FDG PET in Bladder Cancer.
For staging: An estimated 17% change was noted in management effect, based on 12 patient studies (Table 9).
For recurrence: An estimated 17% change was noted in management effect, based on 12 patient studies (Table 9). Because management effect for both staging and recurrence is based upon the same single study of 12 patients, results should be interpreted with caution.
Gastroesophageal Cancer

Disease Background (Gastric Cancer).
Cancer of the stomach, also called gastric cancer, is a disease in which cancer cells originate from the tissues of the stomach. Cancer of the distal half of the stomach has been decreasing in the United States since the 1930s. However, in the last 2 decades, the incidence of cancer of the cardia and gastroesophageal junction (upper half of the stomach) has been rising rapidly. The incidence of this cancer, especially in patients younger than 40 y, has increased dramatically. In localized distal gastric cancer, >50% of patients can be cured. However, early stage disease accounts for only 10%–20% of all cases diagnosed in the United States. The remaining patients present with metastatic disease in either regional or distant sites. The overall survival rate in these patients at 5 y ranges from almost no survival for patients with disseminated disease to almost 50% survival for patients with localized distal gastric cancers confined to resectable regional disease. Even with apparent localized disease, the 5-y survival rate of patients with proximal gastric cancer is only 10%–15%. Although the treatment of patients with disseminated gastric cancer may result in palliation of symptoms and some prolongation of survival, long remissions are uncommon. Radical surgery represents the standard form of therapy with curative intent. Lesser surgical procedures also may play important roles in palliative therapy for patients with gastric cancer. Neoadjuvant or postoperative chemotherapy and/or radiation therapy are under clinical evaluation.
Disease Background (Esophageal Cancer).
Carcinoma of the esophagus is increasing rapidly in frequency in the west, with the rise most apparent in patients with adenocarcinoma of the esophagus. Much of the increase is thought to be related to reflux esophagitis and Barrett’s esophagus (conditions in which acid from the stomach damages the esophagus), but the exact cause is uncertain. Adenocarcinoma of the esophagus is now more prevalent than squamous cell carcinoma in the United States and western Europe, with most tumors located in the distal esophagus. Esophageal cancer is a treatable disease but is rarely curable. The overall 5-y survival rate in those cases amenable to surgery ranges from 5%–20%. The occasional patient with very early disease has a better chance of survival. Primary treatment modalities include surgery alone or chemotherapy with radiation therapy. Combined modality therapy (chemotherapy plus surgery or chemotherapy and radiation therapy plus surgery) is under clinical evaluation.
Case Example (Gastric Cancer).
A 35-y-old patient underwent surgery for gastric cancer. At the time of surgery, a portion of the stomach was removed around the tumor site. During surgery, it was noted that lymph nodes near the stomach also were involved. The patient therefore underwent chemotherapy to treat for spread of the gastric cancer. A CT scan was performed after 6 mo and showed questionable enlargement of lymph nodes in the abdomen. An FDG PET scan was ordered to determine whether the lymph nodes seen on the CT scan were in fact consistent with tumor involvement. The FDG PET scan (Fig. 10) shows several areas of focal increased FDG accumulation in the midabdomen (arrow), confirming tumor recurrence.
Why Did FDG PET Help?
FDG PET confirmed that the questionable findings on CT scan, in fact, were likely to be tumor. Sometimes the CT scan can show lymph node enlargement when no tumor has recurred. In the case shown, there was likely to be tumor recurrence, and the patient now could be managed with the maximal information in hand.
Case Example (Esophageal Cancer).
A 59-y-old man with known esophageal cancer was referred for FDG PET scanning before surgery. CT demonstrated the presence of the primary tumor but no spread of disease. FDG PET showed uptake in the primary tumor (Fig. 11B, lower arrow) and a lymph node near the trachea (Fig. 11A, arrow, and 11B, upper arrow). The esophageal cancer had spread beyond the esophagus.
Why Did FDG PET Help?
FDG PET showed that the cancer had spread beyond the esophagus. Esophageal surgery alone, therefore, was not the best way to manage this patient.
Key Management Issues.
-
Staging for possible spread of tumor
-
Assessing for recurrence
Summary of Evidence for FDG PET in Gastroesophageal Cancer.
For diagnosis: An estimated 14% change was noted in management effect, based on 99 patient studies with 276 lesion sites (Table 10).
For staging: An estimated 20% change was noted in management effect, based on 229 patient studies (Table 10).
For diagnosis/staging: An estimated 14% change was noted in management effect, based on 109 patient studies (Table 10).
Hepatocellular Cancer
Disease Background.
Adult primary liver cancer is a disease in which cancer cells start to grow in the tissues of the liver. People who have hepatitis B or C or cirrhosis, a disease of the liver, are more likely than other people to get adult primary liver cancer. Primary liver cancer is different from cancer that has spread from another place in the body to the liver. Hepatocellular carcinoma is a relatively uncommon tumor in the United States, although its incidence is rising. It is the most common cancer in some other parts of the world. Hepatocellular carcinoma is potentially curable by surgical resection, but surgery is the treatment of choice for only the small fraction of patients with localized disease. Prognosis depends on the degree of local tumor replacement and the extent of liver function impairment. Therapy other than surgical resection is best administered as part of a clinical trial. Hepatocellular carcinoma is associated with cirrhosis in 50%–80% of patients. Five percent of patients with cirrhosis eventually develop hepatocellular cancer, which is often multifocal. Childhood liver cancer, also called hepatoma, is a rare disease in which cancer cells are found in the tissues of a child’s liver. Two types of cancer (hepatoblastoma and hepatocellular cancer) start in the liver and are identified by the way the cancer cells look under a microscope. Hepatoblastoma is more common in children younger than 3 y and may have a genetic cause. The overall survival rate for children with hepatoblastoma is 70% but is only 25% for hepatocellular carcinoma.
Case Example.
A patient presented to his doctor with vague abdominal symptoms. The work-up, which eventually included a CT scan, revealed that the patient had enlarged lymph nodes near the portal region of the liver. An FDG PET scan was ordered to further evaluate for tumor. The scan revealed uptake of FDG within a focus in the right lobe of the liver (Fig. 12, center). No other foci were present, indicating that the tumor was confined to the liver. The patient went on to have an appropriate surgery for localized hepatoma.
Why Did FDG PET Help?
FDG PET indicated that the tumor was localized and that the patient was a candidate for surgery.
Key Management Issues.
-
Distinguishing between cirrhosis and hepatoma
-
Assessing response to treatment and differentiating tumor from necrosis, edema, and scarring
-
Identifying multifocal lesions
Summary of Evidence for FDG PET in Hepatocellular Cancer.
For staging: An estimated 60% change was noted in management effect, based on 20 patient studies (Table 11).
Muscle and Connective Tissue Tumors

Disease Background.
Adult soft tissue sarcoma is a disease in which cancer cells are found in the soft tissue of part of the body. The soft tissues of the body include the muscles, connective tissues (tendons), vessels that carry blood or lymph, joints, and fat. The prognosis for a patient with adult soft tissue sarcomas depends on several factors, including the patient’s age and the size, histologic grade, and stage of the tumor. Factors associated with a poorer prognosis are age older than 60 y, tumors >5 cm, and high-grade histology. Although low-grade tumors usually are curable by surgery alone, higher-grade sarcomas (as determined by the mitotic index and the presence of hemorrhage and necrosis) are associated with higher local treatment failure rates and increased metastatic potential. Soft tissue sarcomas are rare in children and adolescents. There are many different kinds of soft tissue sarcoma, depending on the soft tissue in which the cancer begins. Rhabdomyosarcoma is the most common type of childhood soft tissue sarcoma. It begins in muscles around the bone and can be found anywhere in the body.
Case Example.
A 41-y-old man had surgery and radiotherapy, first for a liposarcoma in the right thigh and 3 mo later for a solitary metastasis in the abdomen. He developed recurrent disease within the right thigh and was referred for FDG PET scanning. The FDG PET scan showed focal increased metabolism within the right thigh (Fig. 13A, B, and C), indicative of recurrent disease, surrounded by diffuse metabolism secondary to inflammation after surgery. High metabolism of FDG was also noted within lung metastases (Figs. 13C and D).
Why Did FDG PET Help?
The FDG PET scan indicated lung and mediastinal metastases, in addition to local disease in the thigh. This meant that the patient would not benefit from treatment of the thigh region alone and would likely require chemotherapy.
Key Management Issues.
-
Following up sarcoma treatment
-
Grading sarcoma
-
Separating benign from malignant masses
-
Selecting biopsy sites
-
Assessing extent of sarcomas
Summary of Evidence for FDG PET in Muscle and Connective Tissue Tumors.
Management change data for diagnosis and staging and other applications are not directly available from the literature (Table 12).
Pancreatic Cancer

Disease Background.
Pancreatic carcinoma is common in the United States, with approximately 30,000 patients each year diagnosed with pancreatic adenocarcinomas. Patients with inflammatory processes in the pancreas (pancreatitis) but no cancer can sometimes have high FDG uptake that is indistinguishable from cancers and, thus, must be differentiated from patients with cancer. FDG PET is being applied increasingly in pancreatic cancer diagnosis. Considering the very poor prognosis of pancreatic carcinomas, PET’s greatest role may prove to be in helping to characterize masses appearing in the pancreas, as opposed to more general tumor staging. This is an active area of current investigation.
Case Example.
A 52-y-old woman with a calcified pancreatic mass on CT (Fig. 14A, arrow) was referred for FDG PET scanning because of rising blood tumor markers. No uptake of FDG was seen within the mass (Fig. 14B). The patient was treated conservatively, under the assumption that she had inflammation of the pancreas (pancreatitis). Follow-up over 2 y with CT revealed no changes, indicating that FDG PET was correct and no tumor existed.
Why Did FDG PET Help?
FDG PET demonstrated that there was no pancreatic tumor, sparing the patient pancreatic surgery.
Key Management Issues.
-
Differentiating chronic pancreatic masses from cancer
-
Staging nodal and liver metastases
-
Assessing response to chemotherapy
Summary of Evidence for FDG PET in Pancreatic Cancer.
For diagnosis: An estimated 50% change was noted in management effect, based on 26 patient studies (Table 13).
For diagnosis/staging: An estimated 43% change was noted in management effect, based on 65 patient studies (Table 13).
For staging: An estimated 36% change was noted in management effect, based on 33 patient studies (Table 13).
For recurrence: An estimated 53% change was noted in management effect, based on 19 patient studies (Table 13).
For monitoring response: An estimated 16% change was noted in management effect, based on 19 patient studies (Table 13).
Prostate Cancer

Disease Background.
Prostate cancer rates increased 141.8% between 1973 and 1994. In 1998, new prostate cancer cases totaled 184,500, or, in other terms, one new case every 3 min. Prostate cancer continues to be the most frequently occurring malignancy (aside from skin cancers), representing 29% of all new cancer cases in American men. One out of every six men is at lifetime risk for prostate cancer. Approximately every 13 min, a life is lost to prostate cancer in the United States. African-Americans have the highest prostate cancer incidence rates in the world, exceeding those for white males in the United States by 34%. Prostate cancer mortality rates are two times higher for African-American men than for white American men.
Case Example.
A 75-y-old man, who was diagnosed with prostate cancer, was followed by blood levels for prostate specific antigen (PSA, a prostate tumor marker). A rising PSA was followed up with a CT scan (Fig. 15, left), which revealed lymph node involvement in the pelvis near the removed prostate. An FDG PET study confirmed what was seen on CT and, in addition, showed spread of cancer into the abdomen and chest (Fig. 15, middle and right).
Why Did FDG PET Help?
FDG PET helped because it showed that the cancer had spread to distant sites and that local radiation to the pelvis alone was not likely to benefit the patient.
Key Management Issues.
-
Further evaluation of equivocal bone lesions found with conventional imaging
-
Differentiating benign from malignant lesions in bone
-
Assessing treatment response when lesion is imaged initially
-
Identifying metastatic disease in soft tissue
Summary of Evidence for FDG PET in Prostate Cancer.
Management change data for staging patients are not directly available from the literature (Table 14).
Renal Cell Cancer

Disease Background.
Renal cell cancer, also called renal adenocarcinoma or hypernephroma, can often be cured if diagnosed and treated when still localized to the kidney and to immediately surrounding tissue. The probability of cure is directly related to the stage or degree of tumor dissemination. Even when regional lymphatics or blood vessels are involved with tumor, a significant number of patients can achieve prolonged survival and probable cure. When distant metastases are present, disease-free survival is poor, although occasionally, patients will survive after surgical resection of all known tumor. Because a majority of patients are diagnosed when the tumor is still relatively localized and amenable to surgical removal, approximately 40% of all patients with renal cancer survive 5 y. Occasionally, patients with locally advanced or metastatic disease may exhibit indolent courses lasting several years. Late tumor recurrence many years after initial treatment occurs occasionally. Renal cell cancer is one of the few tumors in which well-documented cases of spontaneous tumor regression in the absence of therapy exist, but this occurs very rarely and may not lead to long-term survival. Surgical resection is the mainstay of treatment of this disease. Even in patients with disseminated tumor, locoregional forms of therapy may play an important role in palliating symptoms of the primary tumor or of ectopic hormone production. Systemic therapy has demonstrated only limited effectiveness.
Case Example.
A 59-y-old man with a history of metastatic renal cell cancer and left kidney removal developed left-sided flank pain. Abdominal-pelvic CT was negative on initial review (Fig. 16, bottom row). FDG PET revealed a focus in the apex of the right lung and in the left flank (Fig. 16, top row, arrows). Because of the abnormality in the region of the left flank, the CT was reviewed again and a mass located in the posterior abdominal wall was found. Biopsy revealed metastasis from renal cell cancer.
Why Did FDG PET Help?
FDG PET showed a lesion missed on CT and also showed that the renal cell cancer had spread to the lungs. The patient, therefore, could be managed better with systemic therapy and, because of the spread of the disease, was not likely to do well.
Key Management Issues.
-
Detecting metastatic disease
-
Assessing response of metastases to chemotherapy
-
Determining nature of renal masses
Summary of Evidence for FDG PET in Renal Cell Cancer.
Management change data for diagnosis and staging and other applications are not directly available from the literature (Table 15).
Testicular Cancer

Disease Background.
Cancer of the testicle, a rare type of cancer, is a disease in which cancer cells are found in the tissues of one or both of a man’s testicles. Cancer of the testicle is the most common cancer in men 15–35 y old. Men who have an undescended testicle (a testicle that has never moved down into the scrotum) are at higher risk of developing cancer of the testicle. This is true even if surgery has been performed to place the testicle in the appropriate place in the scrotum. Prognosis and choice of treatment depend on the stage of the cancer and the patient’s general state of health.
Case Example.
A 27-y-old man with testicular cancer had his left testicle removed. An abdominal CT scan indicated an enlarged lymph node in the lower abdomen. A CT-guided biopsy was performed but did not reveal cancer. An FDG PET scan was ordered to make sure the biopsy was not wrong. The scan showed a focus of activity in the abdominal lymph node (Fig. 17, arrows), suggesting cancer spread. A repeat biopsy confirmed tumor in the abdominal lymph node site.
Why Did FDG PET Help?
FDG PET showed that the biopsy was wrong, and, in fact, tumor was present in the abdomen. Furthermore, the tumor did not appear to have spread elsewhere. Knowing the presence of tumor in that region changed management for the patient, who would have received only testicular surgery but now could receive additional treatment for spreading testicular cancer.
Key Management Issues.
-
Monitoring response to treatment
-
Staging of primary disease
-
Assessing residual mass
-
Further evaluating raised markers
Thyroid Cancer

Disease Background.
Cancer of the thyroid is a disease in which cancer cells are found in the tissues of the thyroid gland. People who have been exposed to large amounts of radiation or who have had radiation treatment for medical problems in the head and neck have a higher chance of getting thyroid cancer. The cancer may not occur until 20 y or longer after radiation treatment. The four main types of cancer of the thyroid are: papillary, follicular, medullary, and anaplastic. The chance of recovery depends on the type of thyroid cancer, whether it is only in the thyroid or has spread to other parts of the body (stage), and the patient’s age and overall health. Some types of thyroid cancer grow much faster than others. Although thyroid cancer is relatively uncommon, it is nonetheless the most common malignancy of the endocrine system. Differentiated tumors (papillary or follicular) are highly treatable and usually curable. Poorly differentiated cancers (medullary or anaplastic) are much less common but aggressive, metastasize early, and have a much poorer prognosis. The incidence of this malignancy has been increasing over the last decade. The prognosis for differentiated carcinoma is better for patients younger than 40 y and who have no extracapsular extension or vascular invasion. Age appears to be the single most important prognostic factor. Thyroid cancer commonly presents as a cold nodule within the thyroid gland. The overall incidence of cancer in a cold nodule is 12%–15% but is higher in patients younger than 40 y.
Case Example.
A 62-y-old patient underwent surgery of the left thyroid for thyroid cancer. Routine yearly monitoring revealed elevated blood levels of calcitonin. A CT scan was ordered and was normal. An FDG PET scan revealed increased FDG accumulation in the neck (Fig. 18, arrows), which was confirmed by biopsy to be residual thyroid cancer.
Why Did FDG PET Help?
FDG PET identified the source of the rising tumor marker and thereby allowed removal of residual thyroid cancer.
Key Management Issues.
-
Further evaluation when whole-body 131I scan is negative but thyroglobulin (Tg) levels are rising in a patient with known differentiated thyroid cancer
-
Further evaluation for medullary thyroid cancer when rising calcitonin level and initial imaging with dimercaptosuccinic acid V, octreoscan, or metaiodobenzylguanidine is negative.
Summary of Evidence for FDG PET in Thyroid Cancer.
For staging: An estimated 22% change was noted in management effect, based on 60 patient studies (Table 17).
For diagnosis/staging: An estimated 9% change was noted in management effect, based on 58 patient studies (Table 17).
For recurrence: An estimated 53% change was noted in management effect, based on 21 patient studies (Table 17).
Unknown Primary Tumor

Disease Background.
Detection of the unknown primary lesion is very difficult. In many cases, patients present with obvious metastatic disease, often adenocarcinoma, in which the location of the primary lesion may never be found. In some cases, knowledge of the primary site is important, because the type of treatment may vary (e.g., breast cancers are more responsive to some treatments than are renal cancers). This knowledge also can be helpful in resection or treatment for cure of the primary lesion and metastases (e.g., head and neck cancers). FDG PET is useful in locating primary tumors after metastatic disease has appeared in regional lymph nodes. FDG PET is being applied increasingly in the search for unknown primary lesions. This application is still in evolution, but FDG PET should be considered strongly in the work-up of the unknown primary.
Case Example.
A 49-y-old woman presented with lymph node enlargement in the neck. Physical examination, CT, and mammography performed twice all failed to reveal the source of the primary cancer. An FDG PET study showed that the primary cancer was in the left breast (nonpalpable). The lymph node involvement was not seen in the sections shown in Figure 19.
Why Did FDG PET Help?
FDG PET identified the source of the patient’s cancer when no other study could do so, thereby allowing the patient to be treated appropriately for breast cancer with spread to the lymph nodes.
Key Management Issues.
-
Identifying primary site to determine treatment and evaluate for possible resection
Summary
Table 20 is a summary of results from the literature search on FDG PET in cancer.
FDG PET in Cancer: Summary of Results of Literature Search
CARDIAC APPLICATIONS
Myocardial Viability
Disease Background.
A key issue for imaging is to determine whether a given portion of the heart is viable. This means looking at areas of the heart that are not functioning properly and determining whether tissue is still alive and can recover if the blood supply is restored by revascularization. This is a biochemical question. Biochemists and biologists have shown that glucose is a protective substrate to the heart when blood flow is limited. FDG PET helps to determine viability, because those areas of the myocardium that are viable will have glucose metabolism. On the other hand if the myocardial muscle is dead, it will not have any glucose metabolism. The patient whose myocardial muscle demonstrates no glucose metabolism will not benefit from having blood supply re-established to the muscle. Such a patient would need medical therapy or a heart transplant. About 35% of coronary artery disease patients who receive bypass surgery or angioplasty to revascularize the heart do not show improvement in cardiac function because the affected tissue is not reversible (i.e., is dead).
Case Example.
A 57-y-old patient with a previous heart attack was evaluated by echocardiography (echo), which showed that the patient’s left ventricular ejection fraction (percentage of blood ejected from the heart during cardiac cycle) was compromised at 35% (normal >55%) and that wall motion was abnormal. An FDG PET cardiac study was requested to evaluate for viable, reversible myocardium. The PET image on the left in Figure 20 was obtained by using 13N ammonia in a study of blood perfusion to the heart. 13N ammonia has been approved by the U.S. Food and Drug Administration for imaging blood flow in the heart. The donut-like structure is the heart muscle, and the chamber it encloses is the left ventricle. The defect (arrow) seen toward the right side of the donut is an area of compromised blood flow. The image on the right in Figure 20 is the FDG PET glucose metabolism study, and it clearly shows FDG metabolism in the same area that is compromised with regard to blood flow. This patient, therefore, would be likely to benefit from revascularization (bypass surgery to restore blood to a portion of the heart). This is referred to as a mismatch pattern (i.e., low blood flow with high glucose metabolism).
Why Did FDG PET Help?
FDG PET showed that the patient had viable myocardial tissue, which, if blood flow could be restored, could return the function of the heart closer to normal. The patient, therefore, could avoid a heart transplant by undergoing bypass surgery instead. This patient underwent bypass surgery. The ejection fraction returned to 50% and the wall motion to normal levels.
Key Management Issues.
-
Determine whether patients with ischemic heart disease and symptoms of congestive heart failure are best treated with coronary artery bypass surgery, cardiac transplantation, or conservative medical therapy
Summary of Evidence for FDG PET in Myocardial Viability Assessment.
Myocardial viability studies with FDG PET should be performed in patients with ischemic heart disease and impaired left ventricular function who are potential candidates for coronary revascularization (Table 21).
Presence of myocardial viability as determined by FDG PET predicts functional improvement, improved daily life activity levels, and improved survival after revascularization.
NEUROLOGICAL APPLICATIONS
Dementia Work-Up

Disease Background.
Dementia is defined as loss of memory and at least one other area of complex behavior sufficient to interfere with day-to-day function. The magnitude of the problem is increasing, and it is estimated that 5% of the population older than 65 y and up to 25% of the population older than 80 y has some form of dementia. Causes of dementia include degenerative changes (e.g., Alzheimer’s disease, Pick’s disease, Parkinson’s disease, Huntington’s disease), vascular insufficiency, trauma, endocrine changes, and other causes. Metabolic changes in the brain have been shown to precede structural changes by at least 5 y. Treatment for the degenerative forms of dementia, such as Alzheimer’s, is improving with the use of cholinesterase inhibitors and treatment options continue to grow. The diagnosis of early Alzheimer’s disease and its differential diagnosis from other organic dementias or the benign effects of aging remain clinically difficult today. PET with FDG has been shown to provide an accurate and positive differential diagnosis of Alzheimer’s and of other forms of organic dementias. In some ways, the diagnosis of dementia is similar to that for cancer in the separation of benign from malignant disease. In the case of dementia, it is the separation of benign from organic degenerative disease.
Case Example.
A 67-y-old man presented with a 3-y history of progressive loss of memory and day-to-day function and a clinical diagnosis of possible Alzheimer’s. A brain MR image showed no anatomic indications of disease. An FDG PET scan was ordered to evaluate for possible Alzheimer’s disease. Shown in the left column in Figure 21 is a normal FDG PET scan from a 64-y-old man. In the right column is the FDG PET scan from the patient in this case. Two representative slices are shown from each individual. The right column clearly shows low FDG metabolism in the back portion of the brain (arrows) in the parietal and temporal regions. This hypometabolism pattern is consistent with Alzheimer’s disease.
Why Did FDG PET Help?
FDG PET established with a high degree of accuracy that the patient’s symptoms werethe result of Alzheimer’s disease and not other causes of dementia. The diagnosis of Alzheimer’s disease was confirmed 6 y later at autopsy.
Key Management Issues.
-
Early diagnosis of dementia versus benign memory loss
-
Differential diagnosis of dementia from frontotemporal disease, diffuse Lewy bodies, or cerebrovascular diseases
-
Differentiation from pseudodementia/depression (This is a dementia-like state that is caused by depression and not Alzheimer’s disease.)
Summary of Evidence for FDG PET in Dementia Work-Up.
Primary neurodegeneration is the most common process underlying dementia, and Alzheimer’s disease alone accounts for approximately two-thirds of cases. Regional cerebral metabolic patterns reflect pathophysiologic changes in brain that will lead to Alzheimer’s disease, even before they give rise to symptoms. In addition to the diagnostic value FDG PET may have in evaluation of dementia, it can also serve as a prognostic tool to determine the likelihood of deterioration of mental status during the years after scanning, thereby facilitating planning by the patient and family members. Although results have varied, depending in part on the severity and diagnostic mix of patients, nearly all studies designed to assess the accuracy of FDG PET in the diagnosis of dementia have found sensitivity for Alzheimer’s disease to be >90%, with specificity typically approximating 75% (range, 67%–97%). Meeting the challenge of accurately identifying minimally affected patients to allow them to reap the greatest potential therapeutic benefits requires making the diagnosis with a high degree of sensitivity and overall accuracy at the earliest possible stage of disease. The consistently high sensitivity of FDG PET in patients with even mild impairment makes it well suited for assisting with that task (Table 22).
Seizure Work-Up
Disease Background.
Epilepsy is a common condition, with a prevalence in the population of about 1 in 200 people. Several abnormalities within the brain can lead to abnormal “synchronous firing” of neurons, causing a seizure. Depending on which part of the brain is epileptogenic, seizures will have different outward appearances. In a grand mal seizure, all extremities move as a result of abnormal neuronal firing, which spreads within the brain to cause a diffuse motor seizure. Imaging of all types helps to locate abnormalities within the brain, and, when coupled with electroencephalography (EEG, scalp electrical signal monitoring), can help to manage epilepsy patients. Many patients can be controlled well on medications. Patients who have seizures despite having tried several medications are referred to as patients with intractable seizures. In these patients, identifying the source of the seizure within the brain often can lead to surgery that can stop or reduce the seizures. Imaging, including FDG PET, can play an important role in determining whether a patient is a candidate to be operated on for seizure control. The alternative (invasive electronic monitoring) requires putting electrodes into the brain parenchyma or meninges, with attending morbidity and mortality.
Case Example.
An 11-y-old boy, diagnosed with epilepsy at age 7, had been treated with medications for 4 y. During the last year, he had continued to have seizures, even with a change in antiseizure medications. An FDG PET scan was ordered to evaluate for the possible source of the seizure. MRI showed no structural abnormality. The FDG PET scan (Fig. 22) showed moderate-to-severe hypometabolism (lower than normal glucose utilization) in the right parietal, posterior, frontal, occipital, and temporal lobes (arrows) in the interictal period (i.e., between seizures).
Why Did FDG PET Help?
PET showed eleptogenic tissue in the localized brain. Surgery was performed to resect the dysfunctional tissue. The child, after surgery, was seizure free.
Key Management Issues.
-
Diagnosis of partial epilepsy (MRI negative)
-
Localization of seizure focus
-
Prediction of surgical outcome (prognosis)
Summary of Evidence for FDG PET in Seizure Work-Up.
In patients who have medically intractable epilepsy, neurosurgery to resect epileptogenic foci can decrease or eliminate seizure episodes and reduce neurologic impairment resulting from recurrent seizures and/or high doses of anticonvulsants. Patients with complex partial seizures, particularly those who have EEG evidence of a temporal lobe focus but inconclusive findings on MRI, often are referred for functional brain imaging to assess interictal metabolism. PET with FDG can identify epileptogenic zones through localization of hypometabolic brain tissue interictally. Interictal FDG PET has been demonstrated to be as useful for presurgical planning in most patients with temporal lobe epilepsy as the more logistically cumbersome ictal SPECT or more invasive EEG monitoring with depth electrodes. Patients with unilateral foci of hypometabolism identified by PET have been found in numerous studies to have a high likelihood of benefiting from neurosurgery, regardless of whether invasive electrode monitoring is also undertaken. Patients can thus be saved risks and costs otherwise incurred with invasive monitoring. Further study is needed to define more specifically the role of depth electrodes and surgical therapy in patients with findings of bilateral hypometabolism (Table 23).
SUMMARY
For a summary of all FDG PET literature searched, see Table 24.
APPENDIX A. LITERATURE SEARCH CRITERIA AND DATA ANALYSIS METHODS
Literature Search Criteria
The literature search was performed using the databases Medline/Healthstar 1993–2000 and Biosis Previews 1993–2000 for articles and abstracts published from January 1993–June 2000. All key word combinations, including FDG PET, PET, and specific oncologic, neurologic, and cardiac applications were searched. Printed copies of The Journal of Clinical Positron Imaging (1998–2000) and The Journal of Nuclear Medicine abstracts (1996–2000) also were used. Only articles/abstracts in English were used, with the exception of a few English abstracts of non-English-language articles that provided complete information. Both dedicated PET and newer low-cost PET technology (for example, coincidence imaging) studies were included.
The only exceptions to our search time period occurred in the neurological and cardiac application categories. Specifically for myocardial applications, the Medline search extended back to 1986, with a focus on literature assessing viable myocardium. For dementia and seizure workup, the Medline search extended back to 1980, with respective foci on literature assessing accuracy in diagnosing individual patients with dementia and on literature assessing PET performance with respect to evaluating potential candidates for neurosurgery.
All literature that was not clear with respect to methods and/or reporting was excluded. Furthermore, any article/abstract that reported on a study with five or fewer individuals also was excluded. A total of 775 articles/abstracts were retrieved from the literature for our review. Approximately 8 articles could not be obtained from interlibrary requests to outside libraries. The data analysis used 473 unique articles/abstracts (specifically 151 abstracts and 322 articles), and 302 were excluded as per the inclusion/exclusion criteria. The spreadsheets listed a total of 561 article/abstract entries, of which 17 were repeated across several spreadsheets to which they were applicable and 71 were repeated within spreadsheets in multiple applications.
Inclusion Criteria.
(1) Abstracts and articles reporting data within which sensitivity (sens), specificity (spec), positive predictive value (ppv), negative predictive value (npv), accuracy (acc), and management change (mgmt) values were either partially or fully listed or could be partially or fully derived for FDG PET imaging in the 22 different oncologic areas, cardiac viability area, and dementia and seizure work-up areas. In addition, some studies (e.g., seizure) were listed with FDG PET contributions to clinical issues without accompanying accuracy data. Only data with stated or derived total patient studies or total lesions were incorporated into the weighted averages. In those instances in which CT data were found in the PET literature satisfying the inclusion criteria, these were also listed.
(2) Oncologic studies drawn from the period January 1993–June 2000; dementia and seizure studies from January 1980–June 2000; and cardiac studies from January 1986–June 2000.
(3) Response-to-treatment articles were included in the spreadsheets where a 2 × 2 table could be created from the reported data for: responders/nonresponders versus increased FDG/decreased FDG. In those instances in which a 2 × 2 table could not be formulated, the article was excluded.
Note that three articles were also included that provided no numerical information about FDG PET accuracy but had some useful features, which are described in the comments field. These articles, therefore, have no bearing on the weighted averages summarizing all the literature data. These studies by Bischoff et al. (46), Holthoff et al. (197), and Rozental et al. (354) were all part of the monitoring response application.
Exclusion Criteria.
(1) Case reports, review/tutorial articles, and studies with 5 or fewer patients.
(2) Articles not in English. However, abstracts in English of articles not in English but with relevant information were included.
Data Analysis
Data analysis was performed using simple weighted averages. Therefore, studies with more patients were weighted more than studies with fewer patients to arrive at estimates of the sensitivity, specificity, and, when possible, management changes. Weighting is the easiest method to use on such a large number of studies, each of which may or may not present a full 2 × 2 table of outcomes. No attempt was made to perform a formal meta-analysis.
In instances in which articles and abstracts included data for multiple categories (e.g., diagnosis/staging/recurrence), the entire article entry was listed in each of the three individual categories (diagnosis, staging, and recurrence) to preserve the entirety of a study’s reporting and to represent that study’s contribution to data for that category for both this report and possible future analyses that might be looking for all references including data for a given category (e.g., specifically for recurrence.) Only data relevant to a specific category was used in the weighted average for that category (e.g., in calculating the weighted average in the recurrence category, only the recurrence portion of the article’s data was used, even though data for diagnosis also may have been listed).
The number in the total patient studies column sometimes exceeded that in the total number of patients column for a given entry line (e.g., in instances in which patients may have had multiple FDG PET scans). For each line entry of data, the total patient studies or total lesions were listed upon which the 2 × 2 table was based for calculating a given line of data (e.g., if 58 patients had 62 scans from which the true positive (TP), true negative (TN), false positive (FP), and false negative (FN) values were counted, 62 was listed for total patient studies).
In those instances in which articles/abstracts had data broken down for various reported subgroups (e.g., mediastinal and hilar lymph nodes or lymph nodes <1 cm), total patient studies for each subgroup would be listed (as explained above), but often these subgroups would have overlapping patients. In terms of the data analysis, when a given study provided overall values in addition to listing various subgroup values, the overall value was used in the weighted average. When an overall value was not listed, the subgroup data was weighted in by the total patient studies value from which it was generated (or by total lesions, if listed by lesions). The only exceptions occurred in the lung cancer spreadsheet/staging section in the four articles by Baum et al. (36), Tatsumi et al. (424), Ryu et al. (356), and Marom et al. (290). When these studies reported subgroup values for the full patient study count multiple times, the subgroup values were averaged and weighted into the weighted average formula by the total patient studies for one group only.
APPENDIX B. DATA ANALYSIS SUMMARY
Because the default analysis (analysis 1) of our 22 spreadsheets listed all the literature values used that included multiple listings of abstracts/articles both within spreadsheets (e.g., both in the diagnosis/staging and staging applications of a particular cancer) and across spreadsheets, some overlapping of patient studies occurred. To analyze the effects of listing the broad view of the literature values used we performed 5 additional data analyses to study the effects on PET sens/spec/acc by selecting out certain studies according to the following criteria:
(1) Analysis 1 (default analysis): Included all literature used that had some overlap of total patient studies within and across spreadsheets.
(2) Analysis 2: Used data from only those studies that reported jointly the sensitivity and specificity values for PET and PET accuracy values that included one other PET statistical ratio column (e.g., reported PET accuracy and also PET sensitivity).
(3) Analysis 3: Repeated the default analysis, including data from only full research articles. All abstracts were specifically excluded.
(4) Analysis 4: Repeated analysis 2, including data from only full research articles. All abstracts were excluded.
(5) Analysis 5: Pooled sens/spec/acc values for PET across all available studies that provided TP/TN/FP/FN values for each cancer and for all cancers together. This is a formal pooling analysis, using data from each study to construct a large 2 × 2 table. Note that all four cells (TP/TN/FP/FN) must be available, that is, information for patients both with and without disease is required. Therefore, some studies used in some of the weighted averaging subanalysis formulations (e.g., reporting sens only) could not be included in the pooling subanalysis, and thus slight discrepancies exist between the article subsets used in the weighted averages and those used in the pooling.
(6) Analysis 6: Repeated the default analysis excluding all data from nondedicated PET machines (e.g., coincidence imaging).
Results are provided in Table 25.
APPENDIX C. ABBREVIATIONS LEGEND
Abdom: abdominal
Abst: abstract
Acc: accuracy
Activ: activity
Addit: additional
Adenocarc: adenocarcinoma
Adjuv: adjuvant
Adrenalect: adrenalectomy
Adv: advanced
AFP: serum alpha-fetoprotein level
AIDS: acquired immunodeficiency syndrome
ALNDs: axillary lymph node dissections
Amen: amenable
Amput: amputations
Anat: anatomical
Andro: androgen
Antibod: antibodies
Antiestr: antiestrogen
Art: article
Aspir: aspirate
Assessmt: assessment
Asymp: asymptomatic
Autop: autopsy
Av: average
Avdmajsurg: avoid major surgery
BCNU: carmustine
Behav: behavioural
Biochem: biochemical
Biop: biopsy
Bne: bone
BPH: benign prostatic hyperplasia
Br: brain
Brst: breast
BS: bone scintigraphy
BTH: bilateral temporal hypometabolism
B/w: between
C: calcitonin
Ca: cancer
Calcit: calcitonin
Calcs: calculates
Cam: camera
Carcin: carcinoma
Cathet: catheterization
CDM: conventional diagnostic methods
CEA: carcino-embryonic antigen
Cerv: cervical
Chem: chemistry
Chemo: chemotherapy
Chemohormonother: chemohormonotherapy
Chemoradio: chemoradiotherapy
Chge: change
CHOL: 11C choline
Cholang: cholangio-pancreaticography
CI: conventional imaging
Classif: classification
Clin: clinical
Clinstge: clinical stage
Cm: centimeter
Cnfrm: confirmation
CNS: central nervous system
CoDe-PET: coincidence detection PET
Cognit: cognitive
Colonosc: colonoscopy
Compar: comparison
Concom: concomitant
Concord: concordance
Conn: connective
Cont: continued
Contrad: contradictory
Conv: conventional
Corr: correctly
Correl: correlation
CR: complete response
Craniot: craniotomy
Crse: course
CT: computed tomography
CUP: cancer of unknown primary
Cur: curative
CYT-356: capromab pendetide
Cytol: cytology
Cytopath: cytopathology
DAR: differential absorption ratio
Decis: decision
Decr: decrease
DedPET: dedicated PET
Def: definite
Degen: degenerative
Dem: dementia
Detect: detect
Diff: different
Differen: differentiated
Dimens: dimensions
Dis: disease
Discrep: discrepancy
Dissect: dissection
Dissem: disseminated
Dist: distant
Ds: days
DUR: dose uptake ratio
Dwnstge: downstage
Dx: diagnosis
Ea: each
EEG: electroencephalographic
E.g.: for example
Elev: elevation
Endos: endosonography
Endosc: endoscopic examination
Entero: enteroclysis
Epil: epilepsy
Epilept: epileptogenic
Equiv: equivocal
ER+: biopsy-proved estrogen receptor-positive
Esoph: esophageal
Eval: evaluate
Evid: evidence
Ex: exam
Excis: excisional
Exp: experience
Explor: exploratory
Ext: extension
Extrathor: extrathoracic
Fav: favorable
Fd: found
FDG: 2-[F-18]Fluoro-2-Deoxy-D-Glucose
Fm: from
FN: false negative
Fn: function
FNA: fine-needle aspiration
FNAB: fine needle aspiration biopsy
FP: false positive
Ga: gallium
Gastro: gastroesophageal
Gastros: gastroscopy
GCI: gamma camera coincidence imaging
Gde: grade
Gluc: glucose
Gp: group
GRD: gross residual disease
Gynecol: gynecological
H&N: head and neck
HCC: hepatocellular carcinoma
HD: hodgkin’s disease
Hep: hepatic
Hepatocell: hepatocellular
Hi: high
Hippocamp: hippocampal
Hist: history
Histol: histology
Histopath: histopathology
Horm: hormone
Hypertherm: hyperthermic
Hypometab: hypometabolic
Hypopharyng: hypopharyngeal
Ident: identify
Imag: imaging
IMLN: internal mammary lymph node
Immed: immediate
Imprvemt: improvement
IMT: 123I-Iodo-alpha-methyltyrosine
Inconclus: inconclusive
Incorr: incorrectly
Incr: increased
Indeterm: indeterminate
Indic: indicative
Individ: individual
Info: information
Init: initiate; initial
Insuffic: insufficient
Interict: interictal
Intracran: intracranial
Intract: intractable
Involv: involvement
IORT: intraoperative radiation therapy
Irrad: irradiated
IS: immunoscintigraphy
Isol: isolated
Kn: known
Lapar: laporatory
Laparat: laparotomy
Laryng: laryngeal
Lateraliz: lateralization
Les: lesion
Lft: left
LN: lymph node
Lo: low
Loc: local
Locat: location
Locoreg: locoregional
Lowabd: lower abdomen
LT: long term
Lumpect: lumpectomy
Lymphad: lymphadenectomy
Majdwn: major downstaging
Majmgmt: major management
Majupstg: major upstaging
Malig: malignancy
MALT: mucosa-associated lymphoid tissue
Mar: marrow
Mastect: mastectomy
Meas: measurable
Mediast: mediastinal
Mediastinos: mediastinoscopy
Melan: melanoma
MET: 11-Cmethionine
Met: metastatic
Metab: metabolism
Mets: metastases
Mgmt: management
MH: M. Hodgkin
MIBG: metaiodo-benzylguanidine
MIBI: 99mTc-Methoxyisobutylisonitrile
MI-CPS: medically intractable complex partial seizures
Mindwn: minor downstaging
Minmgmt: minor management
Minupstg: minor upstaging
Misc: miscellaneous
MM: mammography
Mo: month
Mod: moderately
Modals: modalities
Mon: monitor
Morphol: morphologic
MR: magnetic resonance
MRD: minimal residual disease
MRI: magnetic resonance imaging
MTC: medullary thyroid cancer
N: total number of patient studies or lesions
N staging: nodal staging
NC: no change
ND: new disease
Necros: necrosis
NED: no evidence of disease
Neg: negative
Neurodeg: neurodegenerative
NHL: non-Hodgkin’s lymphoma
Nk: neck
No: number
Nochge: no change
Noneupstg: none upstaged
NPV: negative predictive value
Nr: near
Nsclc: non-small cell lung cancer
Nt: not
Observ: observation
Occ: occupying
Oper: operable
OPET: other PET
Ophthal: opthalmologic
Osteo: osteomyelitis
Otolaryn: otolaryngologic
Ovar: ovarian
Overstgd: overstaged
P53: p53 protein expression of HCC
Pancr: pancreatic
Papill: papillary
Paramalig: paramalignant
Part: partial
Pathol: pathology
Pblms: problems
Pca: prostate cancer
PCS: positron coincidence scintigraphy
PD: progressed disease
Pelv: pelvic
Perfsurg: perform surgery
Perfus: perfusion
Persist: persistent
PET: positron emission tomography
Phys: physical
Polychemo: polychemotherapy
Poor: poorly
Potent: potentially
PPV: positive predictive value
PR: partial response
Preop: preoperative
Prim: primary
Prob: probably
Proced: procedure
Progress: progressing metastases; progression
Prolif: proliferative
Prost: prostate
PSA: prostate specific antigen
Pt: patient
Pt gp: patient group
Pts: patients
Pulm: pulmonary
RA: research article
Rad: radiation
Rad lymphad: radical lymphadenectomy
Radiog: radiography; radiographic
Radiol: radiological
Recur: recurring
Recurr: recurrence
Ref: reference
Refrac: refractory
Relap: relapsed
Resect: resectable
Resid: residual
Resis: resistant
Restage: restaging
Retroper: retroperitoneal
Retrosp: retrospective
Rev: review
RIT: radioimmunotherapy
RNB: radionuclide bone scintigraphy
Rpts: reports
Rspnse: response
Scint: scintigraphy
Sclc: small cell lung cancer
Scn: scan
SEEG: stereo-EEG
Sens: sensitivity
Ser: serial
Signif: significantly
Simult: simultaneously
Sites: lesion sites
Sl: slight
Smplg: sampling
SNB: sentinel node biopsy
Sonog: sonography
Sp: space
Spdsht: spreadsheet
Spec: specificity
SPECT: single-photon emission CT
SPET: single photon emission tomography
SPN: solitary pulmonary nodule
S.t.: short-term
Std: standard
Stereo biop: stereotactic biopsy
Stg: stage
Strat: strategy
STS: soft tissue sarcomas
Subclin: subclinical
Subgps: subgroups
SUR: standardized uptake ratio
Surf: surface
Surg: surgery
Susp: suspected
Suspic: suspicious
SUV: standardized uptake values
Symp: symptom
Sys: system
Sz: seizure
TCB: trucut biopsy
Temp: temporal
Tg: thyroglobulin
Theor: theoretical
Ther: therapy
Therap: therapeutic
Thor: thoracic
Thorac: thoracotomy
Thyrd: thyroid
Thyroglob: thyroglobulin
Tiss: tissue
T: L ratio:tumor-to-normal liver ratio
TLE: temporal lobe epilepsy
TN: true negative
Tot: total
TP: true positive
Trtmt: treatment
TTNA: transthoracic needle aspiration
Tum: tumor
Ultim: ultimately
Understgd: understaged
Unil: unilateral
Unk Prim: unknown primary
Unnecess: unnecessary
Upstge: upstage
Uptke: uptake
US: ultrasonography
Uter: uterine
UTH: unilateral temporal hypometabolism
Util: utilization
Vasc: vascular
Vert: vertebral
Vs: versus
W: with
WB: whole body
WBS: whole-body scintigraphy
Wd: would
With: withdrawal
Wks: weeks
Wt: weight
WW: watchful waiting
Y: year
131-I: 131-Iodine
2x2: two by two table of true/false positive values and true/false negative values
18F-FDG: 18F-fluorodeoxyglucose
201Tl SPET: 201Tl single photon emission tomography
201Tl SPECT: 201 Tl chloride single-photon emission CT
#
: number
+
: positive
−
: negative
<: less than
>
: greater than
Case example, lung cancer.
Case example, colorectal cancer.
Case example, melanoma.
Case example, lymphoma.
Case example, head and neck cancer. Reproduced, with permission, from Maisey et al. Atlas of Clinical Positron Emission Tomography. London, UK: Arnold, Hodder Headline Group; 1999.
Case example, breast cancer.
Case example, brain tumor.
Case example, ovarian, cervical, and uterine cancer.
Case example, bladder cancer. Reproduced, with permission, from Maisey et al. Atlas of Clinical Positron Emission Tomography. London, UK: Arnold, Hodder Headline Group; 1999.
Case example, gastric cancer.
Case example, esophageal cancer. Reproduced, with permission, from Maisey et al. Atlas of Clinical Positron Emission Tomography. London, UK: Arnold, Hodder Headline Group; 1999.
Case example, hepatocellular cancer.
Case example, muscle and connective tissue tumors. Reproduced, with permission, from Maisey et al. Atlas of Clinical Positron Emission Tomography. London, UK: Arnold, Hodder Headline Group; 1999.
Case example, pancreatic cancer. Reproduced, with permission, from Maisey et al. Atlas of Clinical Positron Emission Tomography. London, UK: Arnold, Hodder Headline Group; 1999.
Case example, prostate cancer.
Case example, renal cell cancer.
Case example, testicular cancer.
Case example, thyroid cancer. Reproduced, with permission, from Maisey et al. Atlas of Clinical Positron Emission Tomography. London, UK: Arnold, Hodder Headline Group; 1999.
Case example, unknown primary tumor.
Case example, myocardial viability.
Case example, dementia.
Case example, seizure.
FDG PET in Lung Cancer: Results of Literature Search
FDG PET in Colorectal Cancer: Results of Literature Search
FDG PET in Melanoma: Results of Literature Search
FDG PET in Lymphoma: Results of Literature Search
FDG PET in Head and Neck Cancer: Results of Literature Search
FDG PET in Breast Cancer: Results of Literature Search
FDG PET in Brain Tumors: Results of Literature Search
FDG PET in Ovarian, Cervical, and Uterine Cancer: Results of Literature Search
FDG PET in Bladder Cancer: Results of Literature Search
FDG PET in Gastroesophageal Cancer: Results of Literature Search
FDG PET in Hepatocellular Cancer: Results of Literature Search
FDG PET in Muscle and Connective Tissue Tumors: Results of Literature Search
FDG PET in Pancreatic Cancer: Results of Literature Search
FDG PET in Prostate Cancer: Results of Literature Search
FDG PET in Renal Cancer: Results of Literature Search
FDG PET in Testicular Cancer: Results of Literature Search
FDG PET in Thyroid Cancer: Results of Literature Search
FDG PET in Unknown Primary Cancer: Results of Literature Search
FDG PET in Miscellaneous Tumors: Results of Literature Search
FDG PET in Myocardial Viability: Results of Lterature Search
FDG PET in Dementia Work-Up: Results of Literature Search
FDG PET in Seizure Work-Up: Results of Literature Search
Summary of Results of FDG PET Literature Search
Data Analysis Summary
Footnotes
Received Dec. 27, 2000; accepted Dec. 27, 2000.
For correspondence or reprints contact: Sanjiv S. Gambhir, MD, PhD, UCLA School of Medicine, 700 Westwood Plaza, B3-399A BRI, Box 951770, Los Angeles, CA 90095-1770.