Abstract
Whole-body PET with 18F-FDG has proven to be a very effective imaging modality for staging of malignant tumors. This study was performed to evaluate the impact of 18F-FDG PET on staging and managing patients for radiation therapy. Methods: The treatment records of 202 consecutive patients (98 male, 104 female; mean age, 56.9 y; age range, 8–91 y) with different malignant tumors were reviewed. Radiation therapy was intended for all patients. The diagnoses were head and neck tumors (n = 55), gynecologic tumors (n = 28), breast cancer (n = 28), lung cancer (n = 26), malignant lymphomas (n = 24), tumors of the gastrointestinal tract (n = 18), and others (n = 23). Whole-body PET was performed before radiation therapy. The alteration of PET on each patient’s staging and management decisions for radiation therapy were determined. Results: For 55 of 202 patients (27%), PET results changed the patients’ management in radiation therapy. In 18 cases (9%), PET resulted in a cancellation of radiation therapy because of the detection of previously unknown distant metastases (8 patients), additional lymph node metastases (9 patients), residual tumor (6 patients), or the exclusion of active disease (2 patients). In 6 patients, >1 incremental reason was found for cancellation. In 21 PET examinations (10%), PET results changed the intention of radiation treatment (curative or palliative). The radiation dose was changed in 25 cases (12%). A change of radiation volume was necessary in 12 patients (6%). Conclusion: The results of this study show that 18F-FDG PET has a major impact on the management of patients for radiation therapy, influencing both the stage and the management in 27% of patients.
Radiation therapy plays an essential role in the interdisciplinary management of patients with malignancies. Accurate tumor staging is a key prerequisite for choosing the appropriate treatment strategy. For curative radiation therapy, distant metastases have to be excluded and locoregional lymph node metastases must be encompassed within the radiation therapy target volume. Determination of the treatment parameters—for example, target volume and dose—are critical when radiation therapy is considered a main agent for local or regional tumor control (1).
Whereas conventional cross-sectional imaging modalities, such as CT and MRI, are sensitive to morphologic changes, identification of tumor tissue (e.g., in normal-sized lymph nodes) is difficult. Furthermore, morphologic imaging modalities are used to evaluate a given region of the body rather than the entire body. Metastases outside the imaging field are missed. Radiation treatment planning based only on CT or MR findings is likely to miss regions of macroscopic tumor in some patients and lead to the irradiation of unnecessarily large volumes in others. Attempts to improve local disease control with increased radiation doses will be futile if all tumors are not included within the high-dose volume.
Several studies have shown that PET using 18F-FDG detects and stages many cancers with a high diagnostic accuracy. High efficacy of PET has been described in lung cancer, malignant melanoma, lymphoma, colorectal cancer, breast cancer, tumors of the abdomen and pelvis, and head and neck cancer (2–12). Clinical-pathologic correlation studies and meta-analyses have confirmed that PET scanning provides much more accurate staging in malignancies than structural imaging alone (13,14). Whole-body PET scanning frequently can detect previously unsuspected distant metastases, thereby sparing incurable patients from futile treatment protocols (15,16).
Recently, 18F-FDG PET scanning has been reported to have an impact for the planning of radiotherapy in cancer patients (17–23). Therefore, we were interested in determining whether staging with PET before radiotherapy has an effect on radiation oncologists to change the intent and radiotherapy treatment planning.
This study was designed to determine the impact of whole-body 18F-FDG PET on the staging and management of cancer patients from the referring radiation oncologist’s perspective. The treating radiation oncologists designated the initial management plans using clinical and imaging information before obtaining the PET scans. These plans were compared with the management actually delivered after PET scanning.
MATERIALS AND METHODS
Study Design
Between July 1998 and June 2001, we performed whole-body 18F-FDG PET in 202 patients (98 male, 104 female; mean age, 56.9 y; age range, 8–91 y) before radiation treatment. The histologic classification of the tumors was available in all cases. Diagnoses are listed in Table 1. The main diagnoses were head and neck tumors, gynecologic tumors, breast cancer, lung cancer, malignant lymphomas, and gastrointestinal tumors. After conventional staging, radiation therapy in the radiation oncology service at our hospital was intended for all patients. Before planning radiotherapy, all available clinical information, including results of clinical findings, imaging studies, and surgical staging, was reviewed. Lymph nodes were regarded as positive for tumor on CT or MRI if they were >1 cm in size. On the basis of all available information, the treating radiation oncologists determined the clinical stage of each patient’s tumor and proposed an initial management plan for radiation therapy, which was documented in the treatment records. In most patients (n = 179), curative radiation treatment was planned, and, in 23 patients, palliative radiation treatment was planned. The pre-PET staging and post-PET staging were always performed independently before and after the PET study. In addition to conventional staging, whole-body 18F-FDG PET scanning was performed in all patients for staging or restaging mainly to exclude distant metastases before radiation treatment. The PET scans were interpreted with all available clinical information, including CT scans. After obtaining the PET scan, each patient was assigned a post-PET tumor stage, which relied on the results of PET when there was discordance with other imaging studies. Where possible, biopsies or further imaging studies were performed to resolve discrepancies between imaging modalities. During the study period, the high accuracy of PET in our patient population became clear. It was considered unethical not to use clear but unconfirmed PET findings for further management decisions, especially in patients with previously unsuspected extensive locoregional disease or distant metastases. An experienced radiation oncologist compared pre-PET and post-PET tumor stages, and the changes in patient management were determined. In the case of detection of a new tumor load, the therapeutic options could change to no therapy, palliative chemotherapy, or radiotherapy for local tumor control but only with a shortened radiotherapy course.
Changes in Radiation Therapy
PET Imaging
To suppress myocardial glucose utilization and to have a low 18F-FDG uptake in all normal tissues, patients were asked to fast for at least 4 h before undergoing the 18F-FDG PET examination. No patients had a history of diabetes. After arriving at the PET center, the patients received an intravenous injection of 300–400 MBq 18F-FDG and rested for 40–50 min for the organ uptake of 18F-FDG. 18F-FDG was produced in our own radiopharmaceutical laboratory using standard techniques. Before PET scanning, patients were encouraged to void to minimize activity in the bladder due to renal excretion of 18F-FDG. Then, patients were transferred to the table of the PET scanner. At 50–60 min after injection of 18F-FDG, a static whole-body emission PET scan was started to cover the patient from the pelvic floor to the head. Transmission scans were acquired in all patients. 18F-FDG PET scanning was performed using an Advance NXi PET scanner (General Electric Medical Systems, Milwaukee, WI) with an axial field of view of 14.6 cm. Emission scans were obtained with a 4-min acquisition time at every table position, typically requiring 6 or 7 bed positions to cover the entire field of view. After emission scanning a transmission scan was started using 68Ge pin sources rotating around the body. Transmission scans were performed from the head to the pelvic floor of the patient, with a 2-min acquisition time at every table position. Image datasets were reconstructed using standard backprojection techniques with and without transmission correction or iteratively with segmented attenuation correction. Quantitative analysis of 18F-FDG uptake in lesions, such as calculation of standardized uptake value, was not performed for routine interpretation of clinical images.
Treatment Planning and Radiotherapy
Radiation therapy was performed in the radiation oncology service at our hospital. Patients received radiotherapy either for a primary curative or palliative intent. Radiation treatment was performed according to institutional guidelines with a radiotherapy dose necessary for local tumor control (e.g., 50–74 Gy) depending on tumor histology and stage. All patients were irradiated with megavolt photons from a linear accelerator. Radiotherapy treatment planning was CT based and primarily conformal. Patients were treated with a 3-dimensional irradiation technique conforming the dose to the target volume, which was drawn by using the different diagnostic imaging modalities. The information of the PET images for the target volume was included by performing visual fusion. The target volume included the tumor with its microscopic spread, lymphatic drainage, and a safety margin to account for movement of organs and variability in the daily treatment setup.
RESULTS
Changes in Radiation Treatment
For 55 of 202 patients (27%), PET results changed the patient management in radiotherapy relating to dose, volume, or intent (head and neck tumors, 33%; gynecologic tumors, 32%; breast cancer, 25%; lung cancer, 31%; malignant lymphomas, 21%; and gastrointestinal tumors, 22%).
The treatment changes and corresponding diagnoses of all patients are listed in Table 1.
The initial intention of the radiation therapy before PET was defined as curative for 179 patients and as palliative treatment for 23 patients. The treatment strategy was changed because of the results of PET (Table 2): for 48 of 179 patients planned for curative radiotherapy (27%) and for 7 of 23 patients planned for palliative radiotherapy (30%).
Changes in Patient Management After PET
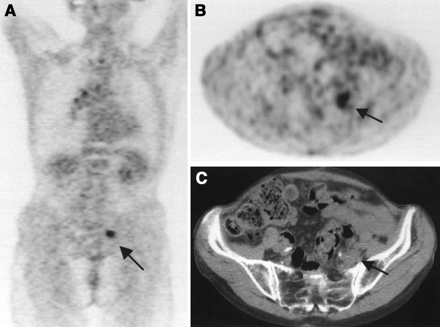
In 18 patients (9%), radiotherapy was not performed after PET because of new findings (alone or combined): distant metastases in 8 patients, additional lymph node metastases in 9 patients, residual tumor in 6 patients, and exclusion of active disease in 2 patients (Table 2; case example in Fig. 1). In 6 patients, >1 incremental reason was found for cancellation: lymph node metastases and distant metastases in 4 patients, lymph node metastases and residual tumor in 1 patient, and lymph node metastases and distant metastases and residual tumor in 1 patient.
A 75-y-old woman after resection of rectal carcinoma. Before PET, curative radiotherapy was planned. Coronal (A) and transverse (B) PET scans show previously unknown iliac internal lymph node metastasis (arrow). Correlating CT scan (C) does not show any metastases. Therapy concept was changed from curative to palliative treatment concept. Therefore, no irradiation but chemotherapy was given. After chemotherapy, PET was performed for restaging. Disappearance of abnormal 18F-FDG accumulation confirmed lymph node metastasis in initial PET study.
In 21 PET examinations (10%), PET results changed the intention of radiation treatment (curative or palliative) (Table 2).
The radiation dose was changed in 25 cases (12%): head and neck tumors, 4%; gynecologic tumors, 14%; breast cancer, 11%; lung cancer, 4%; and malignant lymphomas, 8%. The reasons for this change were the detection of additional lymph node metastases (17 patients), distant metastases (5 patients), and residual tumor (7 patients); for 2 patients dose could be adjusted because of exclusion of residual disease (Table 2).
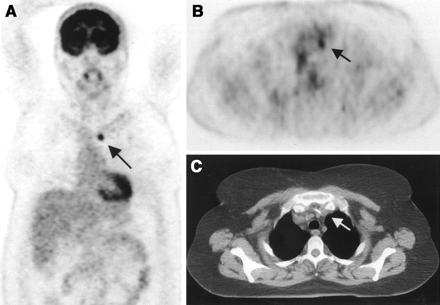
A change of radiation volume was necessary in 12 patients (6%): head and neck tumors, 2%; gynecologic tumors, 11%; breast cancer, 4%; lung cancer, 4%; malignant lymphomas, 13%; and gastrointestinal tumors, 11%. The reasons for this change were the detection of additional lymph node metastases (9 patients), residual tumor (1 patient), and exclusion of metastases in 2 patients because of the results of PET examination (Table 2; case example in Fig. 2).
A 50-y-old woman with lung cancer of right lower lobe. Before PET, primary curative radiotherapy was planned. Coronal (A) and transverse (B) PET scans show previously unknown contralateral mediastinal lymph node metastasis (arrow). (C) Lymph node metastasis is not seen primarily on CT scan. Because of PET, radiotherapy volume was increased.
Results of PET Examinations
The results of all PET examinations are listed in Table 3.
Pathologic Findings of 202 Patients
Recurrent or residual disease was newly detected in 25 PET patients (12%). We categorized the following different malignancies: head and neck tumors, 20%; gynecologic tumors, 11%; breast cancer, 11%; lung cancer, 12%; malignant lymphoma, 4%; and gastrointestinal tract tumors, 6%. A metabolically active tumor was confirmed in 87 of 120 patients (73%) with known morphologically primary or residual tumor. In 20 cases (17%) in which active residual tumor was equivocally reported by other diagnostic methods, PET results were reported as negative. A larger tumor extension—for example, contralateral growth—was discovered in 13 PET examinations (11%). In 12 of 82 cases (15%) without clinical suspicion of recurrence or residual tumor, PET revealed active tumor. In 70 of 82 cases (85%), the absence of tumor was confirmed.
Additional new lymph node metastases were found in 50 cases (25%), lymph node metastases were confirmed in 16 cases, and no lymph node metastases were seen in 136 cases. Previously unknown lymph node metastases were detected by PET in 25% of patients with different malignant tumors: in 42% of patients with head and neck tumors, 21% of patients with gynecologic tumors, 14% of patients with breast cancer, 27% of patients with lung cancer, 8% of patients with malignant lymphoma, and 22% of patients with gastrointestinal tract tumors.
No distant metastases were found by PET in 178 cases. Unknown distant metastases were detected in 24 PET studies (12%). The categories of the different diagnoses are as follows: head and neck tumors, 13%; gynecologic tumors, 14%; breast cancer, 14%; malignant lymphoma, 4%; and gastrointestinal tract tumors, 17%.
Comparison of PET Staging and Conventional Staging
In patients with changes in radiotherapy (n = 55), PET results were compared with the results of conventional staging procedures, including all results of clinical findings, imaging studies, and surgical staging. For 29 of 55 patients, a comparison with other diagnostic methods was performed: The results of different methods were equivalent (both positive or negative) for 6 primary tumors, 16 lymph node metastases, and 6 distant metastases. Findings were confirmed either by histology (n = 7), diagnostic imaging (n = 20), or clinical examination (n = 1). Compared with histology, 1 lymph node metastasis was interpreted as false-positive on PET, but for 7 lymph node metastases that were invisible by imaging and clinical examinations, PET provided additional information. One tumor was not detected by the PET study but was detected by conventional imaging. On the basis of the tumor stage before PET, the prognosis, and the natural course of the malignancies, radiation oncologists interpreted incremental PET findings in 26 patients as tumor positive without further correlation with other imaging studies or histopathology.
DISCUSSION
Whole-body 18F-FDG PET has been proven to be a very effective imaging modality for staging of many malignant tumors, particularly in lung cancer, malignant melanoma, lymphoma, colorectal cancer, and head and neck tumors. Because PET images have a fairly high resolution (<6 mm), even small lesions with an increased 18F-FDG uptake can be detected. This represents a critical advantage of PET over CT and MRI, the conventional cross-sectional imaging modalities. In this study, we described the impact of PET scanning on the management of a population of 202 patients planned for radiotherapy. Over a broad range of clinical indications, PET altered therapy planning in 55 of 202 of patients (27%). Our results are in agreement with previous reports. Kalff et al. (22) reported that in 11 of 28 patients with non-small cell lung cancer (39%) who were candidates for radical radiotherapy, 18F-FDG PET changed the intent or modality of radiation treatment. In a study of Munley et al. (19), PET scans influenced the radiation treatment plans in 12 of 35 patients with lung cancer (34%). Rahn et al. (18) reported that in 16 of 34 patients with head and neck tumors (47%), the radiation treatment plans were altered. In our study, PET scans modified radiotherapy in 31% of patients with lung cancer and in 33% of patients with head and neck cancer.
According to reports in the literature, PET influenced the radiation treatment volume (size and shape of the fields) in a wide range of 26%–65% in patients with lung cancer (17,20–23). In our study, PET altered the radiation volume in only 4% of the patients with lung cancer. This difference is based mainly on different patient selection and on different treatment philosophies. In our study, patients for curative and palliative radiation therapy were included. Mac Manus et al. (23), for example, studied only patients with non-small cell lung cancer for whom radical radiotherapy before PET was intended. Patients with previous radiation therapy or surgical resection were excluded from their analysis. As a result, the treatment volume was increased in 22 of 107 patients (21%) and decreased in 16 of 107 patients (15%) who received a radical therapy after PET. Nestle et al. (20), in their study on the impact of PET for planning of radiotherapy, enrolled patients with lung cancer only associated with an atelectasis. They reported that information provided by 18F-FDG PET would have contributed in 12 of 34 cases (35%) to a change in the radiation field. In our study, 18F-FDG PET resulted in a change of the radiotherapy volume in 36% of patients.
It has been reported that 18F-FDG PET changes the intention of radiation treatment in about 30% of patients with non-small cell lung cancer initially planned for curative therapy (22,23). In our study, we found a change only in 19% of the patients with lung cancer and in 10% for all patients. Because of the change of the treatment strategy, radiotherapy was no longer intended for 18 of 202 patients. Two patients received curative surgery.
Clearly, this study has some limitations. PET was not performed on all patients receiving radiation treatment. Depending on the view of the referring physicians, the risk of metastases or the potential benefit of PET scanning was established for each patient. This study supports the notion that referring physicians at our hospital accept whole-body 18F-FDG PET as an important diagnostic staging modality for the care of their patients. Interestingly, PET detected previously unknown nodal metastases in 25% of all patients and unsuspected distant metastases in 12% of all patients. The highest rates of recurrent or residual tumor and lymph node metastases were found in patients with head and neck tumors. The highest rate of distant metastases was found in patients with gastrointestinal tumors. Patients with malignant lymphoma had minimal overall changes in radiotherapy treatment intention, but radiation volume was changed most often.
We did not analyze whether the changes of the radiation treatment using PET had an effect on the outcomes of these patients. This analysis is difficult to perform because not only the results of improved imaging but also the treatment and prognostic factors will influence the clinical outcomes. A further study imperfection is that only some lesions detected by conventional imaging or PET were confirmed histopathologically. It is well known that 18F-FDG is not specific for tumors but also accumulates in infectious lesions (24,25). We could not ethically justify cytologic or histologic proof of the diagnosis for all lesions identified in our patient population. The radiation oncologists gave high credence to the PET results by taking the pre-PET tumor stage, prognosis, and natural course of the malignancies into account.
Despite these limitations, we believe that our study results are valid, although more clinical data must be accumulated to establish definitively its value for radiation treatment decisions. In an ongoing prospective study, we are currently evaluating the impact of integrated PET-CT on radiation treatment decisions relating to specified tumor types.
CONCLUSION
Our results indicate that whole-body 18F-FDG PET has a considerable impact on patient management in radiotherapy. This study showed that 18F-FDG PET affected staging and management in 27% of patients for whom radiation treatment was intended.
Acknowledgments
This work was supported by the Radium Funds, University Zurich, Zurich, Switzerland.
Footnotes
Received Dec. 26, 2001; revision accepted May 16, 2002.
For correspondence or reprints contact: Hans C. Steinert, MD, Division of Nuclear Medicine, Department of Medical Radiology, University Hospital, Ramistrasse 100, 8091 Zurich, Switzerland.
E-mail: hans.steinert{at}dmr.usz.ch