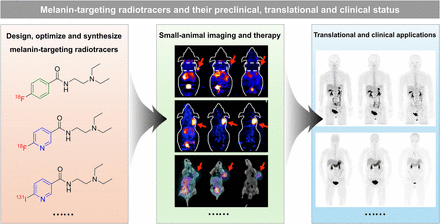
Visual Abstract
Abstract
Melanin is one of the representative biomarkers of malignant melanoma and a potential target for diagnosis and therapy. With advancements in chemistry and radiolabeling technologies, promising strides have been made to synthesize radiolabeled melanin-binding molecules for various applications. We present an overview of melanin-targeted radiolabeled molecules and compare their features reported in preclinical studies. Clinical practice and trials are also discussed to elaborate on the safety and validity of the probes, and expanded applications beyond melanoma are reviewed. Melanin-targeted imaging holds potential value in the diagnosis, staging, and prognostic assessment of melanoma and other applications. Melanin-targeted radionuclide therapy possesses immense potential but requires more clinical validation. Furthermore, an intriguing avenue for future research involves expanding the application scope of melanin-targeted probes and exploring their value.
Melanin is a natural organic biopolymer that is formed by the polymerization of phenolic complexes through quinones (1). Melanin pigment is present in various organs and tissues of humans and animals, such as the retina, substantia nigra, skin, and hair (2). It also accumulates in certain diseases, with melanoma being the most prominent example. Melanoma is a highly aggressive tumor with limited diagnostic and treatment options (3). Exploring melanin as a potential target for diagnosing and treating melanoma shows promise in the management of melanoma. Melanin has various properties, such as light absorption, paramagnetism, and electron exchange (4), and it plays roles in photoprotection, antioxidation, photothermal conversion, and metal chelation (5). Benefiting from these properties of melanin, multimodality imaging and integrative therapy may be useful to target melanin (6). For example, melanin strongly binds metal ions, such as chelated Fe3+, which is observed as T1 hyperintensity on MRI (7). Melanin shows an exceptionally broad spectrum of ultraviolet–visible absorption, which can produce photoacoustic effects for photoacoustic imaging (PAI). It also has high photothermal conversion efficiency used for photothermal therapy (8). However, the hyperintense T1 signal is unspecific, and PAI with photothermal therapy is limited by the penetration depth.
Nuclear medicine techniques use decaying radioisotope–labeled probes to locate and characterize specific biologic processes at the molecular level in vivo and can achieve precise imaging and therapy. The rapid development of chemistry and radiolabeling technology has led to the synthesis of melanin-binding molecules and the use of radionuclides to label molecules used for radiotargeted imaging and therapy, which has generated multiple breakthroughs (9). Advances in genetic bioengineering have led to the establishment of various cells that can be transfected to produce melanin, which serves as an exogenous biomarker when the transfected cell is implanted in the body (10). These developments impel melanin-targeting applications to be more extensive. The explorations of radiotracers and melanin-produced reporter genes have gradually increased and become a hot spot in recent years (11–15).
Here we present an overview of melanin-targeted radiolabeled molecules and compare their features in mice bearing melanoma tumors. Melanin-targeted imaging for exogenous melanin produced by implanted transfected cells is discussed. We focus on melanin-targeting tracers and their preclinical, translational, and clinical status and present our perspective for broadening their applications.
MELANIN-TARGETING MOLECULES FOR NUCLEAR MEDICINE IMAGING
Discovery of Strong Affinities Between Certain Drugs and Melanin
In 1968, chlorpromazine (an antipsychotic derivative of phenothiazine) was the first radiolabeled molecule reported to bind melanin to visualize human malignant melanoma and metastases (16). In 1972, 35S and 14C were used to label chlorpromazine and chloroquine (an antimalaria drug), respectively (17). In the 1990s, other compounds also became known to bind with melanin, such as adiphenine (an inhibitor of nicotinic receptors) and methylene blue (18,19). These molecules display 2 similar chemical structures at physiologic pH: an aromatic or heteroaromatic ring and a protonated amine (Fig. 1). There is always a space linker between the 2 main groups. Further studies focused on the synthesis and selection of the best structures exhibiting these characteristics. The structures and characteristics of typical molecules are summarized in Figure 2 and Table 1 (detailed information is in Supplemental Fig. 1 and Supplemental Table 1 [supplemental materials are available at http://jnm.snmjournals.org]), respectively (20–25).
Example of melanin-binding molecules, showing chemical interactions between benzopyrazine, picolinamide, and benzamide derivatives and melanin fragment.
Structures of representative melanin-targeting molecules for nuclear medicine imaging. Isotopes used to label molecules are indicated in red. Benzene ring structure is shown in green. Nicotinamide and picolinamide ring structures are indicated in blue. Benzopyrazine is shown in peach. Molecules reported in Chinese studies are marked with blue boxes. [123I]MEL008 = N-(2-(diethylamino)ethyl)-5-[123I]iodonicotinamide; [125I]/[18F]4 = 125I- and 18F-labeled N-[2-(diethylamino)ethyl]-4-fluoro-3-iodobenzamide; [18F]DMFB = N-(2-(dimethylamino)ethyl)-4-[18F]fluorobenzamide; [18F]DMPY2 = N-(2-(dimethylamino)ethyl)-5-[18F]fluoropicolinamide; [18F]FPDA = N-(2-(diethylamino)ethyl)-2-[18F]fluoropropanamide; [18F]MEL050 = N-[2-(diethylamino)ethyl]-6-[18F]fluoronicotinamide; 4-[11C]MBZA = 4-11C-methoxy N-(2-diethylaminoethyl) benzamide; BZA-BAT = N-diethylaminoethyl-4-[8-methyl-3-(3-methyl-3-thio-1-azabutyl)-8-thio-2,6-oxoazanonyl]benzamide; ICF15002 = N-(12-ethyl-1-fluoro-3,6,9-trioxa-12-azatetradecan-14-yl)-6-iodoquinoxaline-2-carboxamide; IFNABZA = iodofluoronicotinamide benzamide.
Main Characteristics of Radiolabeled Melanin-Targeting Molecules
Structures with a Benzene Ring and Tertiary or Secondary Amine
In 1991, N-(2-diethylaminoethyl)-4-iodobenzamide (BZA), with a benzamide structure and tertiary amine, was identified and showed a high affinity to melanin pigment (26). When labeled with 125I, it demonstrated high uptake in melanotic tumor tissue of melanoma-bearing mice by scintigraphy. The favorable results led to several investigations into the structure with a benzamide and amine group (27,28). Some reports investigated the binding mechanism of benzamide derivatives to melanin, ruling out receptor–ligand interaction and covalent bond correlation (29,30). Further research showed that electrostatic forces and hydrophobic interactions may play a role in the binding mechanism (Fig. 1). An ionic interaction is produced between the protonated cation on the tertiary amine and the carboxylates of melanin. The aromatic ring binds with the heteroaromatic ring through a π-interaction (18).
To retain the acting groups, studies initially focused on radioiodine-labeling molecules (31–34). However, the detection rate of lesions by these probes is limited to the resolution of SPECT. PET imaging probes were thus further developed (35). Garg et al. (36) and Ren et al. (37) successfully synthesized the same probe, N-[2-(diethylamino)ethyl]-4-[18F]fluorobenzamide ([18F]FBZA). Ren et al. (37) performed in vivo small-animal PET and found significant [18F]FBZA uptake (6.47 ± 2.16 percentage injected dose per gram of tissue [%ID/g]) in B16F10 tumors at 1 h (Fig. 3). However, synthesis of this probe is complex with low radiochemical yield. In later studies, N-(2-diethylaminoethyl)-4-[18F]fluoroethoxybenzamide, [18F]AlF-NOTA-BZA, and N-(2-diethylaminoethyl)-4-[2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy]benzamide were obtained with easier synthesis methods (38–40). N-(2-diethylaminoethyl)-4-[18F]fluoroethoxybenzamide and N-(2-diethylaminoethyl)-4-[2-(2-(2-[18F]fluoroethoxy)ethoxy)ethoxy]benzamide showed higher uptake values (8.66 ± 1.02 and 8.31 ± 1 %ID/g at 1 h, respectively) in tumors containing melanin than did [18F]FBZA but also higher uptake values in tumors without melanin. Thus, the various 18F-labeling benzamide derivatives exhibit different in vivo pharmacokinetics.
Representative images of radiolabeled melanin-targeting molecules. PET/SPECT imaging shows mice bearing melanoma at different time points after injection of 18F-labeled tracers (A) (12,37,45), 125/131/123I-labeled tracers (B) (42,52,80), and 68Ga-labeled tracers (C) (60–62). Red arrows indicate tumors. [123I]MEL008 = N-(2(diethylamino)ethyl)-5-[123I]iodonicotinamide; [125I]40 = N-[2-[ethyl(4-fluorobut-2-ynyl)amino]ethyl]-6-[125I]iodoquinoxaline-2-carboxamide; [18F]DMPY2 = N-(2-(dimethylamino)ethyl)-5-[18F]fluoropicolinamide; 5-[131I]IPN = N-(2-(diethylamino)ethyl)-5-[131I]iodopicolinamide; PCA = procainamide. (Reprinted with permission of (12,45,52,60–62,80) and from (37,42).)
The preceding studies focused on modifying the benzene ring to improve radiochemical yield, melanin-targeting ability, or pharmacokinetic properties. Some researchers focused on the aliphatic amine group. Pyo et al. (13) changed the N-substituents of the amine group from ethyl to methyl to synthesize N-(2-(dimethylamino)ethyl)-4-[18F]fluorobenzamide, which resulted in rapid and prolonged retention in melanoma, as well as the highest uptake among benzamide derivatives (13.00 ± 3.90 %ID/g at 1 h). Liu et al. (41) constructed an aliphatic probe, N-(2-(diethylamino)ethyl)-2-[18F]fluoropropanamide, that contains only a tertiary amino group. Tumor uptake (4.39 ± 0.51 %ID/g at 1 h) was lower than that of [18F]FBZA, but only by an electrovalent bond with melanin, and it quickly decreased to 2.65 ± 0.48 %ID/g at 2 h. This suggests that the aromatic ring structure is necessary for building the melanin-targeted PET probe.
Nicotinamide and Picolinamide Derivatives for Melanin
In addition to benzamide, nicotinamide and picolinamide have the chemical properties for binding to melanin and can be synthesized by a nucleophilic substitution reaction in a single step. Liu et al. (42) prepared a series of iodonicotinamides based on N-2-diethylaminoethyl-4-iodobenzamide, and N-(2-(diethylamino)ethyl)-5-[123I]iodonicotinamide displays high tumor uptake and rapid clearance from the body. It is probable that the hydrophilic character of the pyridine moiety of the nicotinamides versus the aryl moiety of the benzamide analogs contributed to improved urinary excretion of the nicotinamides. Greguric et al. (43) synthesized various structures of 18F-nicotinamide probes, and N-[2-(diethylamino)ethyl]-6-[18F]fluoronicotinamide showed the highest target-to-nontarget ratio (up to 40 at 3 h). Chang et al. (44) reported that [131I]iochlonicotinamide showed rapid and sustained uptake in pigmented melanoma, coupled with consistent improvement in the target-to-background ratio.
Liu et al. (45) synthesized 3 18F-pyridine amides, and N-[2-(diethylamino)ethyl]-5-[18F]fluoropicolinamide ([18F]P3BZA) (46) has the greater advantages, such as higher tumor-to-muscle ratios and good stability in vivo. This tracer, with an additional fluoroalkyl moiety, has high lipophilicity and tends to display nonspecific binding to normal organs, such as high concentrations in the liver (4.71 ± 1.47 %ID/g at 1 h). Researchers optimized the structure with a short-chain polyethylene glycol (triethylene glycol) to generate N-(2-diethylaminoethyl)-5-(2-(2-(2-[18F]fluoroethoxy)ethoxy]ethoxy) picolinamide ([18F]PFPN) (47), which led to reduced liver uptake (2.27 ± 0.45 %ID/g at 1 h) and improved pharmacokinetics (logP = −0.69 ± 0.02) (48). Pyo et al. (12) also changed the N-substituents of the amine group to methyl and synthesized N-(2-(dimethylamino)ethyl)-5-[18F]fluoropicolinamide, exhibiting the highest uptake (24.86 ± 2.30 %ID/g at 1 h) in B16F10 tumors among reported nicotinamide and picolinamide derivatives.
Fluorinated and Iodinated Matched-Pair Radiotracers and Benzopyrazine Derivatives
Based on the highly favorable results using radiohalogenated aromatic and heteroaromatic analogs (49), researchers investigated a new approach consisting of using iodinated and fluorinated matched-pair radiotracers targeting melanin and offering the potential for both diagnosis via SPECT (123I or 125I) or PET imaging (124I or 18F) and therapy (131I). Billaud et al. (50) developed several fluoroaromatic, fluoroheteroaromatic, iodoaromatic, and iodoheteroaromatic derivatives of N-(2-diethylaminoethyl)-6-iodoquinoxaline-2-carboxamide (ICF01012), and 125I- and 18F-labeled N-[2-(diethylamino)ethyl]-4-fluoro-3-iodobenzamides provided good in vitro and in vivo stability and quite similar tumor uptake at 1 h after injection. The researchers also synthesized a series of iodobenzopyrazine derivatives with various side chains bearing fluorine (51,52). The results showed that N-(12-ethyl-1-fluoro-3,6,9-trioxa-12-azatetradecan-14-yl)-6-iodoquinoxaline-2-carboxamide and N-[2-[ethyl(4-fluorobut-2-ynyl)amino]ethyl]-6-[125I]iodoquinoxaline-2-carboxamide exhibited high tumoral uptake and favorable kinetics and thus are good candidates for both SPECT/PET imaging and targeted radionuclide therapy (TRT) of melanoma. Chen et al. (53) created another innovative melanin-targeting probe, [131I]iodofluoronicotiamide benzamide, by combining benzamide with nicotinamide. It exhibited lower initial tumor uptake (5.84 ± 1.80 %ID/g at 1 h) but displayed more stable tumor retention (5.17 ± 1.53 %ID/g at 48 h) than [131I]iochlonicotinamide (13.48 ± 1.77 %ID/g at 1 h and 1.51 ± 0.31 %ID/g at 48 h, respectively) (44).
Melanin-Targeting Molecules Labeled with Radioactive Metal Nuclides
99mTc is inexpensive, convenient, and easily labeled. While considering the preceding results, several 99mTc complexes, based on the structural elements of benzamide (54–56), and benzamide analogs (57) were reported for melanoma. Most showed relatively high radiochemical yield but exhibited limited affinity for the pigmented tumor with moderate tumor uptake. 99mTc-labeled probes ([99mTc]Tc1 and [99mTc]2) with a single tertiary amino group were also investigated but did not display ideal uptake (2.17 ± 0.42, 4.95 ± 1 %ID/g at 1 h) in the melanoma tumors (58,59). The chelating agents have a high hydrophilic property, which can influence the physiologic properties of radiolabeled bioactive molecules.
68Ga is easily obtained from a 68Ge/68Ga generator without the need for a medical cyclotron and has been the subject of attention in recent years. Several molecules labeled by 68Ga were also developed to bind with melanin (60,61). The tumor uptake values of 68Ga-labeled benzamide derivatives were lower than that of [18F]FBZA (6.47 ± 2.16 %ID/g at 1 h) (37). Radiolabeled benzamide derivatives undergo transport mediated by passive diffusion through the cell membrane to interact with melanin in the cytosol. The passive diffusion was positively correlated with the lipophilicity of the compounds. The 68Ga-labeled benzamide derivatives displayed lower logP values, implying fewer lipophilic properties than 18F-labeled benzamide derivatives. These results may indicate that radioactive metal nuclides are not ideal for labeling melanin-targeting small molecules for the use of a hydrophilic chelating agent. Wang et al. (62) investigated a dimer strategy to further improve the tumor-binding capacity. The researchers labeled pyridine-based benzamide dimers with 68Ga to produce [68Ga]Ga-H-2 but with poor tumor uptake (2.89 ± 0.42 %ID/g at 1 h). Lin et al. (63) combined self-assembling peptides with PFPN but obtained only modest enhancements in tumor retention. These findings affirm the conclusion that metallic nuclides are not suitable for labeling melanin-targeting small molecules.
CLINICAL APPLICATIONS OF MELANIN-TARGETING IMAGING
Melanin-Targeting Imaging for Melanoma in the Clinic
Several clinical studies have been conducted to investigate the clinical value of melanin-targeted radiotracers (Table 2) (64–67). [125I]BZA and N-(2-diethylaminoethyl)-2-[123I]iodobenzamide are 2 radiotracers that have been extensively studied. Both showed high sensitivity and specificity in detecting melanoma lesions in early clinical trials (68–70). In a study involving 110 patients with a history of malignant melanoma, [123I]BZA scintigraphy showed high diagnostic efficiency, with a sensitivity of 81%, accuracy of 87%, and specificity of 100% (68). However, in a multicenter phase III clinical study involving 87 participants conducted by Cachin et al. (71) (Supplemental Fig. 2), the sensitivity of N-(2-diethylaminoethyl)-2-[123I]iodobenzamide in diagnosing melanoma metastases was notably lower, at 39%, than the 87% for [18F]FDG. Its relatively poor performance may be attributed partly to the low resolution of SPECT imaging.
Summary of Representative Clinical Studies of Melanin-Targeted Radiotracers
Two 18F-labeled small molecular probes targeting melanin, [18F]P3BZA and [18F]PFPN, were the first to enter clinical trials in China. The safety and tolerance profiles of the 2 probes have been well established in healthy volunteers (72,73). Biodistribution in healthy volunteers revealed that both tracers rapidly clear from the bloodstream, facilitating effective image contrast (Fig. 4). Both [18F]P3BZA and [18F]PFPN showed significant accumulations in the kidneys and bladder, indicating predominant renal clearance. [18F]P3BZA exhibited higher liver uptake than [18F]PFPN at various time points, possibly because of its higher hydrophobic nature.
Representative images of [18F]PFPN and [18F]P3BZA PET in healthy volunteers and melanoma patients (72,73). Maximum-intensity projection (MIP) images were acquired with [18F]PFPN (A) and [18F]P3BZA (B) PET from healthy female volunteers. Moderate [18F]P3BZA uptake was observed in regional obsolete lymph nodes that showed calcification in chest. (C) Man who had surgical resection of choroidal melanoma underwent [18F]PFPN PET/MR and [18F]FDG PET/CT imaging. [18F]PFPN PET demonstrated capability to detect more lesions (indicated by arrowheads and arrows) than [18F]FDG PET. (D) MIP images of melanoma patient showed higher [18F]P3BZA uptake than [18F]FDG in melanoma lesions (arrows) at 60 min after injection. (Reprinted from (72,73).)
In an evaluation of diagnostic efficacy, Zhang et al. (73) conducted a comparative study of [18F]PFPN and [18F]FDG in 21 melanoma patients. The results showed that [18F]PFPN detected more metastases than [18F]FDG and showed excellent contrast, particularly in the brain and liver (Fig. 4). The research group then conducted a study to investigate the prognostic value of [18F]PFPN PET in a cohort of 76 melanoma patients (47). The results revealed that [18F]PFPN PET outperformed [18F]FDG PET in terms of prognostic capabilities for predicting both death and disease progression. Thus, melanin-targeting imaging may be useful to further understanding of melanogenesis in melanoma development, progression, and treatment.
Expanding Applications Beyond Melanoma
Researchers in China have conducted initial investigations into the application of [18F]PFPN PET in tumors beyond melanoma. In a case report (74), [18F]PFPN PET effectively visualized pigmented epithelial adenomas with a size of at least 5 mm in the corpus ciliare that was missed in [18F]FDG PET (Fig. 5), suggesting the imaging potential of [18F]PFPN in lesions other than melanoma that contain melanin. Zhang et al. (75) also evaluated the feasibility of [18F]PFPN PET in clear cell sarcoma. Despite the scarcity of studies, some of which involve a limited number of patients, these findings indicate fresh prospects for the applications of targeted melanin imaging.
Representative [18F]PFPN PET and CT images of pigmented epithelial adenomas and clear cell sarcoma. (A) Patient who reported worsening vision and eye pain for more than 3 mo. MRI revealed 4.1 × 3.7 × 5.0 mm lesion in corpus ciliare with slightly reduced T1-weighted signals. [18F]PFPN showed high activity (SUVmax, 7.1), suggesting melanin expression. Subsequent pathology confirmed pigmented epithelial adenoma (74). (B) Patient with history of clear cell sarcoma surgery underwent [18F]PFPN PET scan for recurrence detection. [18F]PFPN PET found hepatic metastatic lesions < 1.0 cm missed with [18F]FDG (75). T1WI/FS = T1-weighted imaging/fat-suppressed; T2WI/FS = T2-weighted imaging/fat-suppressed. (Reprinted from (74,75).)
MELANIN-TARGETING MOLECULES FOR RADIOTHERAPY
TRT with Small Molecules
The successful use of radiolabeled benzamide and its analogs in melanoma imaging indicates these melanin-binding molecules are promising candidates for TRT. Some of these probes have been assessed for therapeutic efficacy in mice, and these studies are summarized in Supplemental Table 2 (22–25). Joyal et al. (49) synthesized N-(2-diethylamino-ethyl)-4-(4-fluoro-benzamido)-5-[131I]iodo-2-methoxy-benzamide ([131I]MIP-1145). In vivo studies showed that the uptake of [131I]MIP-1145 in melanin-containing SK-MEL-3 human melanoma xenografts remained at 5.91 %ID/g at 24 h. A single dose of [131I]MIP-1145 to mice bearing SK-MEL-3 melanoma led to a striking suppression of tumor growth, with substantial tumor regression observed with multiple doses. [131I]ICF01012 is one of the most extensively studied radiotracers that showed potent antitumor effects in both murine and human pigmented melanoma in therapeutic experiments (76,77). In highly pigmented B16BL6 models, a single dose of 14.8–22.2 MBq of [131I]ICF01012 was sufficient for effective treatment (78). In contrast, less pigmented SK-MEL-3 tumors required 3 doses of 25 MBq to achieve effective radiotherapy (79).
In a study in China, Xu et al. (80) successfully synthesized N-(2-(diethylamino)ethyl)-5-[131I]iodopicolinamide and applied it to treatment of melanoma. Two doses of 18.5 MBq administered to mice with B16F10 melanoma resulted in notable suppression of tumor growth and extended median survival (24 vs. 16 d in control). No hematologic toxicity was observed during the treatment. In investigations of the highest absorbed dose in the liver, blood tests and histopathologic examinations revealed no discernible damage to the liver, underscoring its safety.
TRT with Peptides and Antibodies
Melanin-binding deca- or heptapeptides (4B4 or AsnProAsnTrpGlyProArg) were labeled with 188Re and demonstrated antimelanoma activity (81,82) with relatively quick clearance in tumor tissue. Melanin-binding murine or humanized antibodies, 6D2, 8C3, and h8C3, displayed high affinity for melanin, showing the potential for melanoma treatment, especially when antibodies were labeled with α-emitter 213Bi (83–86). However, there is an antigen barrier during melanin-targeted radioimmunotherapy that hinders deeper penetration of antibodies into the melanoma (87). Therefore, more efforts are required in preclinical investigations for deeper understanding of the complexities involved in melanin-targeted radioimmunotherapy.
Clinical Applications of Melanin-Targeting Therapeutic Probes
In the field of melanin TRT, both antibodies and small-molecule drugs have undergone initial assessments in clinical trials. Mier et al. (27) conducted a pioneering human study using benzo(1,3)dioxolo-5-carboxylicacid(4-(2diethylaminoethylcarbamoyl)-2-[131I]iodo-5-methoxyphenyl)amide and established the safety and efficacy of small molecule–based TRT. Notably, 3 of 5 patients who received doses ranging from 4.3 to 6.6 GBq of benzo(1,3)dioxolo-5-carboxylicacid(4-(2diethylaminoethylcarbamoyl)-2-[131I]iodo-5-methoxyphenyl)amide survived beyond 2 y, a stark contrast to untreated or underdosed patients with an average overall survival of roughly 3 mo, heralding a promising future for benzamide-based TRT. [188Re]Re-6D2 was also investigated in phase Ia and Ib studies to evaluate the safety, pharmacokinetics, dosimetry, and antitumor activity (88). However, transient human antimouse antibody responses were observed in 60% (9/15) of the patients. Additional clinical trials involving melanin-targeted radiotracers are under way (89).
MELANIN AS AN EXOGENOUS BIOMARKER
Melanin-targeted molecules have displayed promising applications in melanoma. However, melanin has multiple characteristics and functions and can be used as a specific biomarker in other fields. Some researchers have used genetic engineering to transfect nonpigment cells so that they produce melanin. This technology broadens the potential of melanin-targeted applications.
Tyrosinase Reporter System
Tyrosinase is the rate-limiting enzyme in the melanin synthesis pathway. Many studies have transfected the tyrosinase gene to nonpigment cells to produce melanin. Because of the various characteristics of melanin, transfected cells that express melanin can be analyzed by multimodality imaging. In 1997, Weissleder et al. (90) transfected the tyrosinase gene into mouse nonpigment cells (fibroblasts and human embryonal kidney cells). Transfected cells had a higher 111In-binding capacity and markedly higher signal intensity in MRI than nontransfected cells. Qin et al. (10) further used [18F]P3BZA as a PET reporter probe for tyrosinase. After transfection of MCF-7 cells, melanin expression was successfully monitored by [18F]P3BZA at the cellular level and in vivo. The system showed high sensitivity for PAI and featured good contrast on T1-weighted images, demonstrating the feasibility of a single reporter for 3-modality imaging. It has the superiority to avoid the complexity of the construction of multiple reporter gene fusions and uncontrollable expression.
In addition to applications in tumor imaging, the multifunctional reporter gene was used in myocardial infarction in a study in China (14). The researchers successfully transferred the tyrosinase gene into bone marrow mesenchymal stem cells (Fig. 6), which were used to treat myocardial infarction. PAI, MRI, and [18F]P3BZA PET 3-modality imaging were used to monitor the survival, distribution, and function duration of the transfected stem cells in the myocardial infarction area. The results demonstrated the myocardial infarction site with clear signals on PET, MRI, and PAI for at least 28 d, suggesting the use of melanin as an exogenous biomarker was a feasible and reliable method.
(A) Scheme of tetracycline-controlled transactivator tyrosinase (TYR) reporter system and molecular imaging applications. [18F]P3BZA PET (B), MRI (C), and photoacoustic (D) 3-modality imaging was used to monitor survival, distribution, and function duration of transfected stem cells in myocardial infarction area (14). Dopa = 3,4-dihydroxyphenylalanine; Dox = doxycycline; MSCs = mesenchymal stem cells; PDT = photodynamic therapy; PTT = photothermal therapy; TetIIP = tetracycline-inducible promoter; Ubi-TetR = ubiquitin promoter–tetracycline repressor. (Reprinted with permission of (14).)
Tetracycline-Controlled Transactivator Tyrosinase Reporter System
Virus-mediated tyrosinase gene transfection into cells may have the risk of changing the growth rate, signal transduction pathways, and other cellular behaviors. An inducible system is an effective method for controlling genetic expression (91). Paproski et al. (92) used the tetracycline-controlled transactivator system to control the expression of the tyrosinase gene, with doxycycline as the inducer. Researchers used this system for inducible expression of the tyrosinase gene in vivo, and the induced melanin-expressing tumors showed strong signals in multiwavelength PAI (93).
A study in China used the inducible system to achieve multimodal imaging of PET, MRI, and PAI (94). Transfected MDA-MB-231 cells produced melanin under the induction of doxycycline, which was detected with sensitivity by [18F]P3BZA and PAI. Theoretically, after being transfected to produce melanin, nonpigment cells can be used in melanin TRT. However, gene editing of cells is complex, time-consuming, and limited by uncontrollable immunogenicity, gene mutation, gene expression time, and gene expression amount. Ongoing development of genetic engineering technology and efforts to improve and optimize these techniques are required for future clinical applications.
EXPERIENCES IN CHINA AND FUTURE PERSPECTIVES
Researchers have been studying probes targeting melanin since approximately the mid-20th century, and these efforts are ongoing. Small molecules, peptides, and antibodies exhibit distinct characteristics and trade-offs in terms of pharmacokinetics and biologic effects. Over the past decade, researchers in China have made substantial efforts in the development and application of melanin-targeting probes, providing findings and insights for further research in this field.
In terms of design, probes based on nicotinamide and picolinamide structures exhibit higher tumor uptake and more desirable tumor retention than benzamide derivates. Research groups worldwide, including those in China, have proved the effectiveness of in vivo imaging and therapeutic efficacies (12,43,48). Furthermore, the development of iodofluoronicotinamide benzamide, containing nicotinamides with benzamides, is a novel approach, and combinations of the 2 structures may be effective (53).
With regard to probe optimization, polyethylene glycolation is considered effective for optimizing pharmacokinetics, as demonstrated by [18F]PFPN. Polyethylene glycolation strategies enhance the probe’s hydrophilicity, reducing uptake in nontarget organs and providing better imaging contrast. To enhance tumor retention, self-assembling peptide strategies may prove somewhat effective (63). Another novel attempt involves dimerizing the melanin-targeting portion to enhance tumor uptake (62). Various strategies have yielded unsatisfactory results, confirming that 68Ga may not be the optimal choice for labeling such molecules. These approaches can offer inspiration for designing therapeutic probes that require enhanced and prolonged tumor uptake, and the effectiveness of these strategies needs validation in probes labeled with isotopes other than radioactive metal nuclides.
Clinical trials for [18F]P3BZA and [18F]PFPN as 18F-labeled melanin-targeting molecule probes have been pioneered in China (72,73). Detailed in vivo studies conducted in healthy volunteers have robustly affirmed the safety and tolerability of these probes. Compared with the traditional imaging agent [18F]FDG, these probes exhibit superior imaging performance, highlighting their significant value in melanoma diagnosis and staging. Furthermore, melanin-targeted imaging has shown potential value in assessing the prognosis of melanoma patients. This suggests that using the imaging parameters of such probes may aid in roughly quantifying melanin and assessing the melanin burden in disease, thereby offering better understanding of the role of melanogenesis in disease progression. Although these results are promising, some patients may have less pigmented or amelanotic melanoma or lesions with diminishing or absent melanin during disease progression and treatment. This underscores the importance of using melanin-targeted imaging probes to select appropriate patients before embarking on melanin TRT.
The imaging capabilities of melanin-targeted probes have been validated for other melanin-containing diseases, such as pigmented epithelial adenomas and clear cell sarcoma (74,75), in preliminary studies. This suggests a broader range of potential applications for melanin-targeted probes beyond melanoma. Another promising application of melanin-targeted probes is tyrosinase reporter gene imaging. This strategy offers the potential for noninvasive, repetitive, long-term tracking of cells, with the prospect of extending applications to cell therapy, such as chimeric antigen receptor T cells.
In summary, preclinical and clinical data collectively support the promising prospects of melanin-targeted probes. Given the unique binding characteristics of these probes to melanin, both well-structured design and appropriate radionuclide selection are paramount in their development. Melanin-targeted imaging holds potential value in the diagnosis, staging, and prognostic assessment of melanoma. The excellent imaging capabilities of melanin-targeted probes also demonstrate significant potential for use in TRT. However, clinical validation remains limited, necessitating further exploration and concerted efforts to transition from bench to bedside. Furthermore, an intriguing avenue for future research involves expanding the application scope of melanin-targeted probes and exploring their value in other domains.
DISCLOSURE
This work was financially supported by the National Natural Science Foundation of China (grants 82372026 and 82030052). No other potential conflict of interest relevant to this article was reported.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.
- 22.↵
- 23.
- 24.
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.
- 33.
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.
- 66.
- 67.↵
- 68.↵
- 69.
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.
- 85.
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- Received for publication October 26, 2023.
- Revision received January 31, 2024.