Abstract
The effect of multiple patient-related factors on the degree of cardiac 18F-FDG uptake was assessed. Methods: Five hundred four consecutive patients undergoing routine 18F-FDG PET/CT studies completed a clinical questionnaire. 18F-FDG uptake was measured as the mean standardized uptake value within the heart delineated on the CT component of the study. Univariate and multivariate analyses assessed the influence of 51 clinical factors on cardiac 18F-FDG uptake. Results: On both multivariate and univariate analyses, cardiac 18F-FDG uptake was significantly lower in diabetics and in patients receiving bezafibrate or levothyroxine. Cardiac 18F-FDG uptake was significantly higher in men, patients younger than 30 y old, fasting duration of <5 h, patients with heart failure, and those receiving benzodiazepines. Conclusion: Cardiac 18F-FDG uptake was lower in patients receiving bezafibrate and levothyroxine and higher in patients receiving benzodiazepines. If further confirmed by prospectively designed studies, manipulation of these drugs may represent tools for optimized PET/CT imaging.
FDG with 18F has been suggested as a tracer to visualize inflammation in atherosclerotic plaques in the large arteries (1,2). To extend this observation to plaques in the coronary arteries, myocardial 18F-FDG uptake must be as low as possible. In patients with thoracic tumors, low 18F-FDG uptake in the heart facilitates detection of malignant lesions that may be otherwise obscured by high myocardial tracer activity.
Glucose metabolism in the heart is influenced by the availability of substrate, myocardial workload, and adequacy of myocardial perfusion (3). Healthy subjects demonstrate variable myocardial 18F-FDG uptake (4). Previous reports have suggested that the degree of cardiac 18F-FDG uptake is related to free fatty acid (FFA) and serum glucose levels (5–8). A prolonged fast, shifting substrate use from glucose to FFA, has been recommended as an approach to reduce myocardial uptake.
It is likely that additional factors may influence 18F-FDG uptake in the heart. This study correlates myocardial 18F-FDG uptake in fasting patients with a group of 51 patient-related clinical variables, aiming to identify additional factors that could be potentially manipulated to intentionally decrease myocardial 18F-FDG uptake and, thus, improve PET/CT imaging for specific oncologic and cardiovascular indications.
MATERIALS AND METHODS
Patient Population
All patients who were >20 y old and who had an 18F-FDG PET/CT examination for known or suspected malignancy over a 4-mo period were invited to enroll in the study. Consenting patients completed a clinical questionnaire. Exclusion criteria were (a) patients who could not provide details regarding drug intake, (b) patients showing paracardiac foci of high 18F-FDG uptake or lesions on CT that could potentially impair a precise delineation of the heart (as described in the image interpretation section), and (c) patients showing misregistration between the PET and CT components of the study. The parameters assessed in this questionnaire and the patients' characteristics are summarized in Tables 1 and 2. The study was approved by the Institutional Review Board.
Demographic and Clinical Patient Characteristics
Medication Intake 24 Hours Before 18F-FDG Administration
Imaging Technique
Patients were instructed to fast for at least 5 h, except for noncaloric fluid intake, before the injection of 370–555 MBq (10–15 mCi) 18F-FDG. Whole-body PET/CT acquisition was started at approximately 60 min after tracer injection using the Discovery LS PET/CT system (GE Healthcare Technologies). Non–breath-hold CT was acquired at 80 mA·s and 140 kV. 18F-FDG PET acquisition was performed with a 4-mm slice thickness and reconstructed iteratively using ordered-subset expectation maximization software. CT was used for low-noise attenuation correction of and fusion with PET data. Fused images were visually analyzed for the presence of misregistration.
Quantitative Measurement of 18F-FDG Uptake in Heart
The heart volume was defined on the CT component by a bullet-shaped volume of interest (VOI), and 18F-FDG uptake was subsequently measured on the PET component inside this VOI. The heart was detected on the CT scout view registered to the maximal-intensity-projection image on PET. After this initial determination, the presumed center and angulations of the short, vertical long, and horizontal axes of the heart were displayed on registered PET and CT slices. The synchronized corresponding PET and CT images were manually recentered and rotated for optimal cardiac delineation. A bullet-shaped region of interest (ROI) was defined over the heart. The size and position of the ROI were then manually adjusted to ensure that the whole heart was included in all 3 views, defined by CT images. Precise registration between CT and PET was verified by synchronized scrolling through all axial slices overlying the heart. The VOI of the whole heart was calculated from the ROIs in the 3 planes.
Standardized uptake values (SUVs) of 18F-FDG in the cardiac VOI were measured using the commercial calculation methods corrected for physical decay and attenuation (Fig. 1).
Quantitation of cardiac 18F-FDG uptake. Volume of heart is defined with detection of cardiac boundaries on CT component of PET/CT study and using a bullet-shaped VOI to measure tracer concentration over whole heart. 18F-FDG uptake is measured on PET component inside CT-defined VOI. (A) Patient with high cardiac 18F-FDG uptake. (B) Patient with low cardiac 18F-FDG uptake. SUVmean = mean SUV.
Statistical Analysis
The nonparametric Mann–Whitney U test was used on univariate analysis to assess differences in cardiac 18F-FDG uptake in relation to the patient-related factors. Variables with frequencies below 2% of the population (found only with respect to intake of 9 of 38 drugs) were not included. The Pearson correlation test was performed for univariate analysis to determine the relationship between cardiac 18F-FDG uptake and continuous parameters, such as age, body mass index (BMI), heart rate, blood pressure, glucose level, time from 18F-FDG injection, and duration of fast. A threshold, designating a significant difference in SUV, was established for the continuous parameters, and the Scheffé test was used to find significant differences between the groups. Multivariate analysis was used to consider the effects of the investigated factors simultaneously. Patients with missing information on any of the variables were excluded from this analysis. Patients with diabetes, receiving oral antidiabetic medication or having blood glucose levels of >150 mg/dL, overlapped among these variables and, therefore, were included as a single group. Thus, multivariate analysis was performed on a final group of 368 patients for whom information regarding all variables was available. Patients with missing information regarding any of the 22 patient-related variables were not included in this particular analysis. A P value < 0.05 was considered statistically significant.
All measurements and statistical analyses were performed using the index of mean SUV (SUVmean) and maximal SUV (SUVmax). Correlation between SUVmean and SUVmax was established using linear correlation.
RESULTS
Patient Characteristics
Five hundred four consecutive studies on 495 patients were evaluated. The demographic and clinical characteristics of the patients are summarized in Table 1. Medication intake during the 24 h before the PET/CT study, in 338 patients (67%), is summarized in Table 2.
Quantitative Cardiac 18F-FDG Uptake
The range of SUVmean-measured cardiac 18F-FDG uptake was 0.6−13.9 (mean ± SD, 3.2 ± 2.3). SUVmax-measured cardiac 18F-FDG uptake ranged between 1.6 and 33.1 (mean ± SD, 8.7 ± 6.4), with a very good linear correlation between SUVmean and SUVmax (r = 0.987).
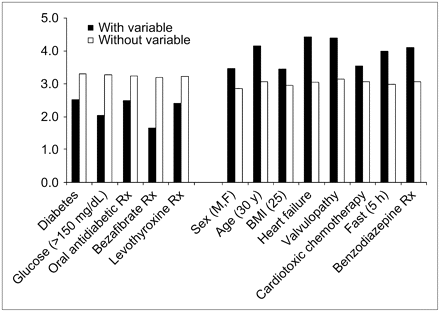
Univariate analysis demonstrated a lower cardiac SUVmean in diabetics (n = 70, P = 0.007) and in patients with glucose levels of >150 mg/dL at the time of tracer injection (n = 43, P = 0.001). Medications associated with decreased 18F-FDG myocardial uptake included oral antidiabetics (n = 53, P = 0.001), the lipid-lowering agent bezafibrate (n = 11, P = 0.001), and the thyroid replacement drug levothyroxine (n = 40, P = 0.028). Variables associated with lower cardiac 18F-FDG uptake are presented in Table 3 and Figure 2.
Variables significantly affecting cardiac 18F-FDG SUVmean uptake (mean values) in univariate analysis. Cardiotoxic chemotherapy indicates regimens containing anthracyclines: doxorubicin, idarubicin, or epirubicin. Rx = treatment.
Patient-Related Variables Associated with Significantly Lower Cardiac 18F-FDG Uptake (Univariate Analysis)
Univariate analysis demonstrated higher cardiac 18F-FDG uptake in men (n = 250, P = 0.003), patients < 30 y old (n = 40, P = 0.004), BMI < 25 (n = 206, P = 0.009), history of heart failure (n = 18, P = 0.050), valvular disease (n = 21, P = 0.004), after cardiotoxic chemotherapy (n = 113, P = 0.049), and duration of fast of <5 h (n = 79, P < 0.001). Among the medications, benzodiazepines were associated with increased 18F-FDG uptake (n = 45, P = 0.003). Factors associated with higher cardiac 18F-FDG uptake are presented in Table 4 and Figure 2.
Patient-Related Variables Associated with Significantly Higher Cardiac 18F-FDG Uptake (Univariate Analysis)
Multivariate analysis demonstrated a lower SUVmean in diabetics (P = 0.015) and in patients taking bezafibrate (P = 0.028) or levothyroxine (P = 0.044). A significantly higher SUVmean was related to age (<30 y, P = 0.001), sex (male, P = 0.008), heart failure (P = 0.013), fast <5 h (P < 0.001), and intake of benzodiazepines (P = 0.022). Results of multivariate analysis are presented in Table 5.
Variables Significantly Affecting Cardiac 18F-FDG Uptake (Multivariate Analysis)
The same statistical analysis was performed for SUVmax as well, showing the same trend, with consistent results compared with those obtained for SUVmean (data not shown).
DISCUSSION
18F-FDG PET of the chest may be impaired by intense tracer uptake in the heart. Variable, inhomogeneous cardiac tracer activity may affect visualization of metabolically active atheromatous plaques in coronary arteries and detection of malignant lesions in paracardiac location. The present study evaluates the effect of 51 factors on 18F-FDG uptake in the heart in a group of 504 consecutive patients undergoing routine 18F-FDG PET/CT studies. 18F-FDG activity was quantified by defining the heart volume on the CT component and measuring 18F-FDG uptake as the SUVmean on the PET component of the study. This method enables uniform and reproducible quantification and overcomes the difficulty of identifying the metabolic boundaries of the heart using only PET slices in cases where cardiac 18F-FDG uptake is scarce or heterogeneous.
Myocardial tracer uptake was found to be significantly influenced by 3 of 29 administered drugs—bezafibrate, levothyroxine, and benzodiazepines—as well as by 5 of 22 patient-related variables.
The decrease in cardiac 18F-FDG uptake was most prominent in the 11 patients receiving the lipid-lowering drug bezafibrate (Table 3; Fig. 2). Bezafibrate reduces serum triglyceride levels by affecting lipoprotein metabolism (9). Altered substrate availability related to intake of this drug, further promoting high FFA levels at the expense of glucose, may result in the decreased 18F-FDG uptake. However, further research is necessary to confirm this hypothesis.
Cardiac 18F-FDG uptake was significantly lower in patients reporting levothyroxine intake. A decrease in myocardial 18F-FDG uptake in patients receiving thyroid replacement therapy has, to the best of our knowledge, not been reported previously. Thyroid hormones upregulate cardiac adrenergic receptors, increase sensitivity to sympathetic stimulation, and increase heart rate. Administration of thyroid hormones also stimulates glucose transport and glycolysis by upregulating glucose transporter-4 (GLUT-4) transcription (10). Hypothyroid rats show a decrease in the expression of glucose transporters and activity of phosphofructokinase-1 (11,12). Although it is assumed that patients taking levothyroxine probably have hypothyroidism, serum thyroxine levels were not specifically measured in this study.
Patients taking benzodiazepines demonstrated statistically significant increased cardiac 18F-FDG uptake. Benzodiazepine receptors are present in the central nervous system and in peripheral tissue, including the heart (13). Benzodiazepine intake significantly lowers 18F-FDG uptake in the brain (14) and may reduce physiologic 18F-FDG uptake in brown fat in the region of the neck (15). Increased 18F-FDG uptake in the heart related to benzodiazepine intake has not been described previously and requires further investigation.
Men had significantly higher cardiac 18F-FDG uptake. This cannot be attributed to the larger size of the male heart because the heart volume is considered when calculating SUVmean. Sex-based differences of regional 18F-FDG uptake in the brain have been previously attributed to differences in perfusion (16–18). It may be hypothesized that current findings could also be due to sex-based genetic differences in the molecular and cellular physiology of the heart (19), with further influence on cardiac glucose metabolism.
Patients < 30 y old were found to have a significantly higher SUVmean, a result that does not agree with a previous report that found no correlation between cardiac 18F-FDG uptake and age (6). These different results could be related to the qualitative evaluation of a smaller group of patients in the previous study. Age has been described as a factor that influences 18F-FDG uptake in different tissues, such as the brain (16) and the large arteries (2). A proposed mechanism for lower 18F-FDG uptake in patients > 30 y old could be the presence of reduced GLUT-4 levels, as described in a rat animal model (20), leading to a proportional decline in uptake of glucose and 18F-FDG.
In addition to the above new data, the present study confirms literature reports with regard to overall cardiac 18F-FDG uptake variability (4), lower uptake in diabetics (21), and higher tracer activity encountered with shorter periods of fasting (5–8). Although patients with heart failure evaluated in our study had higher cardiac 18F-FDG uptake, previous studies have shown conflicting data with regard to this clinical parameter (22,23).
Among the 47 patients with coronary artery disease (CAD), 30 had a history of myocardial infarction. In addition, 292 patients had known risk factors for CAD. These parameters were evaluated since cardiac glucose use is partially related to myocardial perfusion (3). In addition, this subgroup of patients is one of the potential target populations for vulnerable plaque imaging, and finding ways to minimize cardiac 18F-FDG uptake in these patients is of particular relevance. There was no statistically significant relationship between cardiac 18F-FDG uptake and the presence of risk factors for, or history of, CAD (apart from diabetes), a known previous MI, as well as heart rate and blood pressure levels measured at the time of tracer injection.
Limitations of the present study include its observational design, as patients were enrolled consecutively from a pool of subjects undergoing routine PET/CT studies for their cancer care. Individual medication doses were not recorded, and blood levels of FFAs or hormones such as insulin, known to potentially affect cardiac 18F-FDG uptake, were not specifically measured. 18F-FDG uptake measurements included the entire heart and, thus, may contain a certain degree of blood-pool tracer activity in addition to the myocardial uptake. Because similar and consistent results were obtained for SUVmean and SUVmax, it is assumed that the biodistribution of the tracer did not influence current quantitative results.
Reducing myocardial 18F-FDG uptake can be of potential benefit to several patient populations. In cancer patients, decreasing 18F-FDG uptake in the heart can improve detection of malignant lesions in a paracardiac location. In patients with atherosclerotic disease, low cardiac tracer activity using 18F-FDG can improve detection of vulnerable plaques in coronary arteries.
CONCLUSION
Instructing patients to fast for at least 4–6 h before the study is, to date, the only known option to decrease 18F-FDG uptake in the heart. The present study identifies the effects of treatment with bezafibrate, levothyroxine, or withdrawal of benzodiazepines as new additional factors that may be useful—if confirmed by future studies—to achieve low 18F-FDG uptake in the heart.
Footnotes
-
COPYRIGHT © 2007 by the Society of Nuclear Medicine, Inc.
References
- Received for publication August 17, 2006.
- Accepted for publication November 11, 2006.