Abstract
Sarcoidosis is a systemic granulomatous disease of unknown etiology. Cardiac involvement may occur, leading to an adverse outcome. Although early treatment to improve morbidity and mortality is desirable, sensitive and accurate detection of cardiac sarcoidosis remains a challenge. Accordingly, interest in the use of advanced imaging such as cardiac MR and PET with 18F-FDG is increasing in order to refine the clinical workup. Although the field is still facing challenges and uncertainties, this article presents a summary of clinical background and the current state of diagnostic modalities and treatment of cardiac sarcoidosis.
- cardiac sarcoidosis
- inflammation
- positron emission tomography
- [18F]-deoxyglucose
- magnetic resonance imaging
Sarcoidosis is defined by the American Thoracic Society, European Respiratory Society, and World Association of Sarcoidosis and Other Granulomatous Disorders as “a multisystem disorder of unknown cause(s)” (1). The incidence varies according to ethnicity, sex, and region, from 3–10 per 100,000 for Caucasians to 35–80 per 100,000 for African Americans. Scandinavians have a higher incidence than other Caucasians. Women are affected more frequently, and peak incidence occurs below 40 y of age (2).
Some factors have been linked with sarcoidosis, such as environmental or occupational sources (3), infectious causes (4), and genetic predisposition (5), but the etiology remains unclear. A central role in early development and progression is attributed to the immune response and the degree of inflammation, which represent a major target for diagnosis and therapy (6).
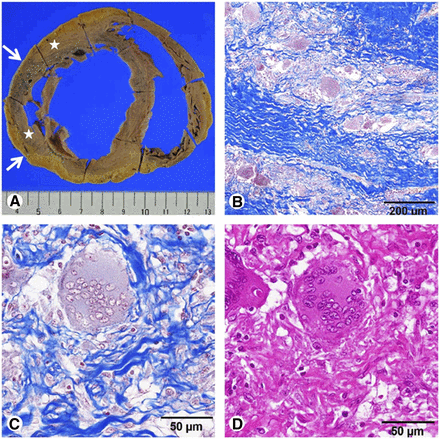
Organ involvement is characterized histologically by noncaseating, nonnecrotic granulomas (Fig. 1) (7). Most frequently, lymph nodes and lungs are involved, but disease can affect any other organ. The likelihood of cardiac sarcoidosis (CS) varies regionally, with a high occurrence in Japan (8,9). CS frequency is debated, with an approximate incidence of 5% based on clinical assessment (10), 27% in autopsy studies (7), and 39% in an imaging study that used cardiac MR (CMR) or PET (11). Even though CS appears to be underdiagnosed clinically, it is considered to be the second leading cause of death by sarcoidosis in the United States (12) and the leading cause in Japan (13).
Histopathology of CS. (A) Macroscopic view of dilated LV wall with thinning (arrows) and scarlike white lesions (asterisks). (B–D) Microscopic view of sarcoid granuloma and interstitial fibrosis stained with Masson trichrome (B and C) and hematoxylin–eosin (D). (Reprinted with permission from (79).)
CLINICAL MANIFESTATION
The clinical manifestation of CS ranges from asymptomatic to sudden cardiac death. It is determined by localization and severity of disease. The myocardium is most frequently affected (6), but granuloma may also affect the endocardium and pericardium (14). The most frequently involved area is the ventricular septum (31.5%), followed by the inferior wall, anterior left ventricle, right ventricle, and lateral left ventricle (14). The left ventricular (LV) free wall, papillary muscles, basal septum, and atrial walls have also been reported as frequent sites of involvement at autopsy (15).
Inflammatory granulomas or postinflammatory scarring may lead to conduction abnormalities, arrhythmias, sudden cardiac death, and congestive heart failure. Involvement of the septum favors conduction abnormalities. Most frequent is a third-degree atrioventricular block, which appears in 23%–30% of cases (15). Banba et al. reported that atrioventricular block develops mainly during the inflammatory phase. Therefore, it has been speculated that early corticosteroid treatment might improve atrioventricular conduction (16). Compared with patients with idiopathic third-degree atrioventricular block, CS patients tend to be younger (17). Therefore, CS should be considered in patients younger than 55 y who present with unexplained persisting second- or third-degree atrioventricular block (18).
Ventricular tachycardia occurs in up to 23% of CS patients and represents the second most frequent clinical finding (16). Ventricular tachycardia is assumed to be reentry-related and attributable to myocardial scar tissue rather than active granuloma (16,19).
In a Japanese study, sudden cardiac death was the initial presentation in 40% of CS patients (20), underlining the need for an accurate tool for early disease detection.
In addition to arrhythmic complications, congestive heart failure is frequent, being observed in up to 73% of patients dying from CS. A variety of reasons, such as extensive myocardial infiltration, right heart failure caused by pulmonary involvement, or valve insufficiency caused by involvement of papillary muscles, contribute to pump failure (21). Accordingly, it has been suggested that CS should be considered in all patients with persistent ventricular tachycardia and idiopathic dilated cardiomyopathy. Generally, cardiac involvement should be ruled out in all patients with sarcoidosis, because of the adverse outcome (18).
THE NEED FOR RELIABLE DIAGNOSIS
Identification of cardiac involvement in sarcoidosis is difficult. A precise and early diagnosis is needed to initiate antiinflammatory therapy and to prevent an adverse outcome (11,22).
The Missing Gold Standard
The missing gold standard is a major problem in the diagnosis of CS. Biopsies are subject to sampling error and cannot be used to rule out CS. To date, the Guidelines of the Japanese Ministry of Health and Welfare (JMHWG), as revised by the Japan Society of Sarcoidosis and Other Granulomatous Disorders in 2006, serve as a worldwide standard for clinical diagnosis of CS (Table 1) (23). But PET, in contrast to CMR or 67Ga scintigraphy, is not included despite its documented sensitivity in detecting CS. There is no uniform consensus on the key aspects of diagnosis and management of CS, as underlined by a recently published Delphi study (24). A consequence of that project was the creation of a proposal for the best practice in diagnosis and management of CS (Table 2).
Revised JMHWG Criteria for Diagnosis of CS (23)
Proposed Diagnostic and Therapeutic Strategy in CS (24)
Clinical Evaluation
CS should be ruled out in all patients with diagnosed extracardiac sarcoidosis and in patients younger than 55 y with unexplained persisting second- or third-degree atrioventricular block or with persistent monomorphic ventricular tachycardia and nonischemic dilated cardiomyopathy (Table 2) (18).
The initial clinical evaluation should include medical history, physical examination, electrocardiography, 24-h Holter monitoring, and an echocardiogram. Symptoms such as chest pain, palpitations, syncope, bradycardia, peripheral edema, dyspnea, and orthopnea may occur but are nonspecific (25,26). Okura et al. showed in a CS cohort that the clinical presentation consists of atrioventricular block (50%), left-sided heart failure (40%), syncope (31%), palpitations (17%), chest pain (14%), and bradycardia (10%) (25). Like the clinical symptoms, electrocardiography changes do allow for conclusions about the extent of disease or the inflammatory activity, but only persistent ventricular tachycardia predicts adverse outcome (21). Signal-averaged electrocardiography can be used to detect intramyocardial conduction delay and identifies CS with 52% sensitivity and 82% specificity using biopsy as a reference (27). Holter monitoring may be suggestive of CS, with 50% sensitivity and 97% specificity using CMR or PET as a reference (11). Freeman et al. confirmed these findings and concluded that Holter monitoring serves as a powerful screening tool to predict a positive CMR or PET scan (28).
Echocardiography abnormalities are common but nonspecific. CS may present as dilated cardiomyopathy, with valvular dysfunction, wall motion abnormalities, wall thickening due to infiltration and edema, or wall thinning, which is located mainly in the septum and associated with scarring (29,30). However, echocardiography detects later stages of CS and is not considered useful for early detection. It has been applied for monitoring of therapy (31) and may predict adverse outcome when LV dilation is present (21).
Endomyocardial biopsy has less than 25% sensitivity in detecting noncaseating granuloma in CS, probably because of the patchy distribution pattern, resulting in sampling errors (7,32). Nevertheless, it is the only method that provides definitive histologic proof. To achieve a higher diagnostic yield, endomyocardial biopsy may be image-guided (33) or electroanatomic mapping–guided (34).
ADVANCED IMAGING
If the initial work-up for CS does not provide a safe rule-out, further testing using tomographic imaging should be performed before a therapeutic decision is made.
CMR
CMR provides high spatial and soft-tissue resolution. It detects the active, inflammatory phase of disease as well as the chronic phase, in which scarring and fibrosis are predominant. The inflammatory phase is characterized by focal wall thickening due to infiltration or edema, combined with wall motion abnormalities seen on T1-weighted (cine) images, increased signal intensity on T2-weighted images, and early gadolinium enhancement (35). The chronic phase predominantly shows wall thinning and delayed gadolinium enhancement, representing myocardial damage, scarring, and fibrosis (Fig. 2) (36). Commonly, the two phases overlap. According to a Delphi study on CMR, delayed gadolinium enhancement was the strongest hallmark of CS (24). Distribution varies and usually does not adhere to coronary vascular territories (37), although it may mimic myocardial infarction (38). The basal septum (39), the basal and lateral segments of the LV (40), and the papillary muscles (6) are frequently involved. In addition to reliable detection of disease, gadolinium enhancement may also be useful to assess the response to steroid therapy (41,42). Patel et al. compared the prognostic value of late gadolinium enhancement on CMR with the criteria of the Japanese Ministry of Health and Welfare in an asymptomatic cohort of 81 patients with biopsy-proven extracardiac sarcoidosis (38). CS was detected in 26% of patients by CMR, but only 12% fulfilled JMHWG criteria. Delayed gadolinium enhancement was associated with adverse events and cardiac death. These results were confirmed by a more recent study, in which 155 patients with extracardiac sarcoidosis underwent CMR and had a follow-up of approximately 2.6 y (36).
CMR imaging in case of CS. (A) Increased thickness (arrows) of mid-basal anterior and anteroseptal walls. (B and C) Transmural hyperenhancement (arrows) of mid-basal anterior, anteroseptal, and right ventricular free walls. (D and E) Excisional biopsy sample of anterior mediastinal lymph node confirming sarcoidosis (fibrosis in left upper quadrant [white arrow]; multiple noncaseating granulomas [black arrow]; no evidence of acid-fast bacilli or fungus). (F and G) Follow-up 3 mo after onset of steroids, showing significantly decreased extent of hyperenhancement (arrows). (Reprinted with permission from (80).)
PET
Increased glucose metabolism is a hallmark of inflammation, because of overexpression of glucose transporters and overproduction of glycolytic enzymes in inflammatory cells (43). Inflammation can be visualized effectively using the glucose analog 18F-FDG and PET.
Under aerobic conditions, most myocardial energy consumption is extracted from oxidation of free fatty acids, followed by glucose and, to a smaller part, amino acids. However, energy production and its relative contribution depend on various factors such as fasting state, neurohormonal conditions, and systemic regulation (44). The Randle cycle states that fatty acid loading suppresses glucose metabolism and vice versa.
It is essential to be aware that myocardial glucose utilization and thus 18F-FDG uptake may be present even under fasting conditions, during which free fatty acids are usually the preferred substrate. Studies have reported that fasting 18F-FDG uptake is inhomogeneous throughout the left ventricle in healthy subjects (45) and in oncologic patients (46), with a regional maximum in inferolateral and basal myocardium, probably because of local differences in substrate use (46). This uptake must not be confused with myocardial inflammation (Fig. 3) and emphasizes the need for an effective strategy to suppress myocyte uptake for a PET-based diagnostic workup of CS.
PET/CT study in subject with idiopathic dilated cardiomyopathy and insufficient suppression of physiologic 18F-FDG uptake. (A) Reangulated images and polar maps of stress (82Rb dipyridamole) and rest (82Rb rest) perfusion show dilated ventricle with inhomogeneous perfusion but no evidence of regional ischemia. Fasting glucose uptake (18F-FDG) maximizes in basal lateral wall and is otherwise diffuse and mild. (B) Whole-body study shows no extracardiac foci of 18F-FDG uptake. HLA = horizontal long axis; MIP = maximum intensity projection; SA = short axis; VLA = vertical long axis.
Currently used strategies for myocardial suppression include prolonged fasting (47), dietary modifications (48,49), and a heparin load before imaging (50). Williams and Kolodny were able to show that a very high-fat, low-carbohydrate, protein-permitted diet suppresses myocardial 18F-FDG uptake more effectively than overnight or 4-h fasting in an oncologic cohort (49). Harisankar et al. compared the effectiveness of myocardial 18F-FDG suppression due to a low-carbohydrate, high-fat, protein-permitted diet with prolonged fasting over 12 h. In that oncologic cohort, dietary restriction better suppressed cardiac uptake than did prolonged fasting (48). Heparin, on the other hand, increases plasma free fatty acid levels and thereby suppresses glucose metabolism. In a Japanese CS study cohort after a fasting period of at least 6 h, 50 units of nonfractionated heparin per kilogram of body weight were injected 15 min before application of 18F-FDG (50). The result was robust suppression of cardiac 18F-FDG uptake. However, a more recent study compared heparin with prolonged fasting of more than 17.5 h and reported that cardiac uptake was more severely inhibited by extended fasting (51). Although there is currently no consensus on the best protocol for suppressing cardiac 18F-FDG uptake, a recently proposed protocol that combines fasting, a fatty meal, and heparin appears to be an attractive solution (52).
If patient preparation is performed correctly, 18F-FDG PET is accurate in the diagnosis of CS. 18F-FDG PET provides functional imaging of inflammatory disease activity and is therefore considered to be beneficial not only for early disease detection but also for therapy monitoring and image-guided biopsy (52). A patchy, focal uptake pattern is most suggestive of CS (Fig. 4). If there is a more diffuse uptake, the physiologic myocardial suppression may have been insufficient and other pitfalls should be considered. These include insufficient myocardial uptake suppression leading to heterogeneous 18F-FDG uptake (maximum in basal and lateral walls); other nonischemic cardiomyopathy or other inflammatory diseases of the heart leading to a substrate shift toward glucose, resulting in (heterogeneous) myocardial 18F-FDG uptake; and myocardial ischemia resulting in sustained regionally increased myocardial 18F-FDG uptake. Diffuse or focally increased uptake of the lateral wall is commonly seen as a normal variant and should not be mistaken for inflammation (53). Maurer et al. were able to show that suppression of total cardiac activity was present in only 9% of an oncologic patient cohort despite adequate fasting (46). Additionally, Israel et al. pointed out that cardiac 18F-FDG uptake was significantly higher in male patients, patients younger than 30 y, patients who had fasted for less than 5 h, patients with heart failure, and patients receiving benzodiazepines (54).
CMR and PET/CT studies in subject with active CS. (A) CMR images, obtained at initial diagnosis, show foci of delayed gadolinium enhancement in basal lateral wall, septum, and apex (arrows). (B) Reangulated PET/CT images and polar maps obtained after cardioverter-defibrillator implantation and steroid therapy show multiple foci of increased fasting glucose uptake (18F-FDG) in basal lateral wall, septum, and apex, suggesting persistent active CS (arrows). Stress (82Rb dipyridamole) and rest (82Rb rest) perfusion images rule out ischemic heart disease and show fixed perfusion deficit in area of active sarcoid in basal lateral wall, consistent with regional tissue damage (arrows). HLA = horizontal long axis; SA = short axis; VLA = vertical long axis.
18F-FDG PET is often combined with a perfusion scan and electrocardiographic gating to rule out coronary artery disease or identify resting perfusion defects suggestive of inflammation-induced tissue damage (52). Normal perfusion and increased focal 18F-FDG uptake represent early CS, whereas abnormal perfusion and increased 18F-FDG uptake more likely represent advanced disease with tissue damage. Scarring, a potential end stage of disease, may result in abnormal perfusion without 18F-FDG uptake (55). After steroid therapy, a small study in 17 biopsy-proven CS patients showed a significant decrease in 18F-FDG uptake whereas perfusion defects remained stable (56).
Also, cardiac assessment can be combined with whole-body imaging to determine the presence and activity of extracardiac sarcoidosis lesions (Fig. 5). Cardiac involvement cannot be predicted by the extent of pulmonary disease (11). Rarely, CS may appear without extracardiac lesions (57). A proposed comprehensive protocol for PET imaging of CS is presented in Table 3.
PET/CT study in subject with CS and extracardiac sarcoidosis. (A) Reangulated images of stress (82Rb dipyridamole) and rest (82Rb rest) perfusion rule out ischemic heart disease. Fasting glucose uptake (18F-FDG) is focally increased in basal inferior wall, consistent with active CS, and is suppressed in other LV walls (arrows). (B) Whole-body study shows multiple extracardiac foci of 18F-FDG uptake in chest, liver, bone, and lymph nodes, consistent with active extensive systemic sarcoidosis. HLA = horizontal long axis; MIP = maximum intensity projection; SA = short axis; VLA = vertical long axis.
Protocol for 18F-FDG PET to Detect CS
In a recent prospective study on 28 patients with biopsy-proven sarcoidosis, 18F-FDG PET/CT influenced clinical management in 63% (47). In a current metaanalysis of 7 studies (164 patients), Youssef et al. reported 89% sensitivity and 78% specificity for CS detection with 18F-FDG PET, compared with JMHWG (53). But like all other diagnostic studies on CS, larger prospective studies are desirable to better define the clinical role of 18F-FDG PET and to determine its value for predicting outcome.
Comparing PET and CMR
Several studies compared late enhancement in CMR and 18F-FDG uptake in PET. While delayed enhancement represents cardiac damage and scarring, 18F-FDG uptake represents active inflammation. Consequently, the mild to moderate correlation between CMR and PET is not surprising (58–60). When CMR and 18F-FDG PET were compared with the JMHWG, CMR had a higher specificity but a lower sensitivity (24,58).
In a study of 57 patients undergoing both CMR and 18F-FDG PET, CMR had a better negative predictive value, indicating it might be superior for ruling out CS (60). The main disadvantage of CMR, however, is the inability to study patients with defibrillators, which are increasingly implanted in CS patients. Another limitation is the inability to use gadolinium to image patients with renal impairment.
Advantages of 18F-FDG PET include the biologic nature of the imaging signal, the potential to identify cardiac and extracardiac sarcoidosis involvement (52), and the feasibility of imaging patients with electrical devices. Another advantage for therapy response or risk stratification may be quantification. According to European Association of Nuclear Medicine and Society of Nuclear Medicine and Molecular Imaging guidelines for 18F-FDG in inflammation, standardized uptake value should be used with caution in this setting (61). But McArdle et al. found higher quantitative 18F-FDG uptake in CS patients with ventricular tachycardia than in those with atrioventricular block and asymptomatic controls (62). And Blankstein et al. concluded in a group of 125 patients that abnormal cardiac PET findings are associated with a higher risk of death or ventricular tachycardia, whereas JMHWG criteria or the ejection fraction are not (63). Nevertheless, therapy monitoring and risk stratifications are areas for which prospective clinical studies are needed to determine the value of imaging.
Disadvantages of 18F-FDG PET include the radiation exposure, the potential pitfalls of inadequately suppressed physiologic cardiac uptake, and the inability to detect smaller regions of myocardial damage. Also, a potential source of error in patients with an implantable cardioverter-defibrillator may be hot-spot artifacts at the lead insertion site on attenuation-corrected images. Although some studies suggested a significant overestimation of SUV (64), others suggested that images in the presence of metallic leads could be interpreted without correction for metal artifacts (65). Evaluation of images without attenuation may be used as an adjunct in case of suspected lead insertion artifacts.
Ultimately, given that cardiac involvement in sarcoidosis is underdiagnosed and associated with an adverse outcome, and given the different strengths and weaknesses and the different biologic signals of PET and CMR, a combination of the two may be beneficial (59,60) and there may be a future role for integrated PET/MR (66).
Other Radionuclide Imaging Tests
Before the advent of clinical PET, myocardial perfusion studies with 201Tl or 99mTc-sestamibi, and 67Ga scintigraphy, were commonly performed to detect and monitor cardiac involvement in sarcoidosis (67). Because of the higher accuracy of PET/CT and CMR, these newer techniques have mostly replaced other radionuclide imaging.
Myocardial perfusion imaging may show patterns of reverse distribution, in which perfusion defects at rest decrease under stress conditions (68). It is believed that reverse redistribution in CS is caused by focal reversible microvascular constriction in coronary arterioles around the granulomas (69). According to the results of a few studies, 99mTc-sestamibi seems to be superior to 201Tl in detecting reverse distribution (67,70).
67Ga scintigraphy detects CS during its inflammatory phase, because of accumulation in inflamed areas. 67Ga scintigraphy can also be used in systemic sarcoidosis. Studies reported a high specificity of almost 100%, whereas sensitivity varied from 0% to 36%, using JMHWG as a reference (56). A weak signal from the long-lived isotope with high-energy photon emission explains the low sensitivity.
THERAPEUTIC OPTIONS AND PROGNOSIS
Imaging-test–driven evidence of active CS is an indication for treatment, because of the poor prognosis and increased risk of sudden death. Even though there are no published clinical consensus guidelines, management aims at controlling inflammation and fibrosis. Basic medical treatment is similar to that for dilated cardiomyopathy, including angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, diuretics, and β-blocking agents (71). According to Milburn et al. (72), corticosteroids attenuate inflammation by reestablishing balance between type 1 and 2 T-helper cell cytokines, which are altered in sarcoidosis.
Beneficial effects of early corticosteroid therapy on LV function have been reported in several studies (16,73). Corticosteroid use has been associated with maintenance of LV function in patients having normal function at diagnosis and with improvement in patients having moderate LV dysfunction. In contrast, LV function in patients who have severe LV dysfunction is unlikely to improve (74). This benefit of early therapy initiation emphasizes the need for early diagnosis. Although corticosteroids remain the mainstay of treatment, there is still uncertainty about optimal initiation, dosage, or duration of therapy and about effects on the clinical course of CS, as they have not been studied in any randomized, prospective trials.
CS may cause reentrant arrhythmias and therefore increase the risk of sudden cardiac death. Hence, there is a trend toward early implantation of prophylactic defibrillators in patients with biopsy-proven systemic sarcoidosis and positive cardiac imaging (71). Even though there are few clinical data, CS is regarded as a class IIa recommendation for an implantable cardioverter-defibrillator (75).
In addition to device implantation, antiarrhythmic agents are often required in patients with CS to eliminate or reduce recurrent ventricular tachyarrhythmia (76). Analogous to medical therapy and cardiac devices, the indication for antiarrhythmic agents is similar to that in nonsarcoidosis patients.
Ultimately, especially in young patients with severe heart failure refractory to medical therapy, heart transplantation needs to be considered carefully (77). Because of the uncommon nature of CS and concomitant disease in other organs, the number of patients undergoing heart transplantation is small (71). But notably, outcomes have been reported to be identical or even superior to those in nonsarcoidosis patients (78).
SUMMARY
The importance of early recognition of cardiac involvement by sarcoidosis is increasingly recognized because of its implications for adverse outcome. 18F-FDG PET/CT and delayed-enhancement CMR are powerful imaging techniques for accurate detection and therapy monitoring. Although protocols for imaging are increasingly well defined for these modalities, the clinical evidence for their accuracy, their relative value compared with each other and with alternative strategies, and the most useful indications is still limited to small-scale observational studies. To address the existing uncertainties and to standardize clinical practice, larger prospective trials including high-end imaging are necessary in CS. It is expected that such trials will better define the value of imaging, and, ultimately, improve outcome through test-driven personalized treatment.
Acknowledgments
The images shown in Figures 3–5 were obtained during the tenure of Dr. Bengel at Johns Hopkins University, Baltimore, MD.
Footnotes
Published online Nov. 14, 2013.
Learning Objectives: On successful completion of this activity, participants should be able to describe (1) the pathophysiology, clinical appearance, and prognostic implications of cardiac sarcoidosis, (2) the practical aspects, typical image patterns, pitfalls, and clinical value of cardiac 18F-FDG PET in sarcoidosis; and (3) the value of 18F-FDG PET relative to other imaging modalities and clinical test results in cardiac sarcoidosis.
Financial Disclosure: The authors of this article have indicated no relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, participants can access this activity through the SNMMI Web site (http://www.snmmi.org/ce_online) through January 2017.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- Received for publication June 27, 2013.
- Accepted for publication September 3, 2013.