Abstract
The purpose of this prospective study was to estimate the effect of 68Ga-labeled prostate-specific membrane antigen (PSMA)–11 PET on the intended management of patients with biochemically recurrent prostate cancer. Methods: Pre- and postimaging surveys were filled out by the referring providers for patients with biochemical recurrence who were imaged using 68Ga-PSMA-11 PET. The inclusion criterion for this study was a prostate-specific antigen (PSA) doubling time of less than 12 mo after initial treatment (NCT02611882). Of the 150 consecutive patients imaged, 126 surveys were completed (84% response rate). The responses were categorized as major change, minor change, no change, or unknown change. Results: There were 103 patients (82%) with disease detected on 68Ga-PSMA-11 PET. On the basis of the survey results, there were 67 patients (53.2%) with major changes in management and 8 patients (6.4%) with minor changes. The proportion of cases resulting in a change in management did not significantly differ by baseline PSA level. In patients with PSA levels below 0.2 ng/dL, 7 of 12 patients had disease detected on 68Ga-PSMA-11 PET, 5 of whom had a major change in management. Conclusion: 68Ga-PSMA-11 PET resulted in a major change in management in 53% of patients with biochemical recurrence. Further studies are warranted to investigate whether PSMA-based management strategies result in improved outcomes for patients.
- molecular imaging
- PET
- biochemical recurrence
- management
- prostate-specific membrane antigen
- prostate cancer
Up to 30% of prostate cancer patients who are treated with definitive local therapy, such as radical prostatectomy (RP) or radiation therapy (RT), have evidence of recurrent or residual prostate cancer (1–3). Recurrence is generally manifested as an increase in prostate-specific antigen (PSA), termed biochemical recurrence (BCR). BCR frequently occurs months to years before there is evidence of disease on standard imaging, thereby limiting the selection of treatment options, since the site of recurrence is not evident. Conventional imaging for staging prostate cancer includes CT, MRI, and 99mTc-labeled phosphate bone scintigraphy (bone scans), all of which have a low sensitivity for recurrent disease, particularly at low PSA levels (4,5).
Several molecular imaging radiotracers, most notably choline derivatives, have been used to increase detection rates in BCR patients, but they have limited sensitivity and specificity at PSA levels of less than 1.0 ng/dL (6,7). Prostate-specific membrane antigen (PSMA) is overexpressed on prostate cancer cells, and its expression appears to increase as its aggressiveness increases, as marked by higher Gleason scores and higher rates of morbidity (8,9). PSMA-targeting PET has demonstrated a much higher sensitivity than conventional imaging (10,11). In particular, the utility of 68Ga-PSMA-11 has been extensively reported over the past 3 y in prostate cancer patients with localized disease or BCR (11–14).
One prospective and 2 retrospective studies have been performed evaluating the effect of 68Ga-PSMA-11 PET on intended management (15–17). The aim of this study was to determine the effect of 68Ga-PSMA-11 PET on the intended management in prostate cancer patients with BCR in a prospective clinical setting. Data on change in management are important in order to support eventual acceptance of 68Ga-PSMA-11 PET by referring clinicians and coverage by insurance companies.
MATERIALS AND METHODS
This study was approved by the local institutional review board, and informed written consent was obtained from all subjects. An Investigational New Drug application was approved by the Food and Drug Administration for this study. From December 2015 to October 2016, 225 patients were enrolled in a prospective study evaluating the use of 68Ga-PSMA-11 PET in the staging of patients with prostate cancer (NCT02611882). The study included 3 cohorts: patients before definitive therapy, patients with BCR after definitive local therapy, and patients with castration-resistant prostate cancer. This report focuses on the 150 patients evaluated for BCR. Patient characteristics are provided in Table 1. Eligible patients had to have undergone definitive local therapy with curative intent and subsequently be found to have BCR. Inclusion criteria required a PSA doubling time of less than 12 mo. Patients were not required to have negative findings on conventional imaging.
Patient Characteristics
68Ga-PSMA-11 Synthesis and Injection
68Ga-PSMA-11 was synthesized as previously reported using a 68Ge/68Ga generator and a manual synthesis module supplied by Isotope Technologies Garching (18). Each synthesis was performed under good manufacturing practices, and quality control was performed for purity, pyrogenicity, and sterility. Patients were injected with 199.8 ± 48.1 MBq (5.4 ± 1.3 mCi) of 68Ga-PSMA-11, and imaging occurred 63 ± 10 min after injection. Twenty milligrams of furosemide were administered to 110 of the patients, given 14 ± 11 min before injection of the radionuclide to minimize the halo artifact caused by scatter overcorrection associated with the high renal and urinary activity (19).
Imaging Protocol
Imaging was performed on either a PET/CT scanner (Discovery VCT; GE Healthcare) or a PET/MRI scanner (3.0-T time-of-flight Signa PET/MRI; GE Healthcare), depending on the referring clinician’s preference. For PET/CT, we imaged from pelvis to vertex, using a 5-min acquisition for the first 3 bed positions (up to the mid abdomen) and subsequent 3-min acquisitions to the vertex. Iodinated contrast material was administered to all patients, and a postcontrast diagnostic CT scan was acquired and used for attenuation correction (249 mA, 120 kV, and slice thickness of 2 mm). PET datasets were reconstructed using 4 iterations, 14 subsets, and a 168 × 168 matrix. The PET transaxial field of view was 620 mm, and axial slices were reconstructed at 5.0 mm thick.
For PET/MRI, we imaged a pelvis and abdomen bed position using an 8- to 10-min acquisition at both bed positions. PET datasets were reconstructed using time-of-flight, ordered-subsets expectation maximization with 2 iterations, 28 subsets, and a 256 × 256 matrix. The PET transaxial and z-axis fields of view were 600 and 250 mm, respectively, and axial slices were reconstructed at 2.8 mm thick. In the pelvis bed position, we acquired a dynamic contrast-enhanced sequence (Dixon-based differential subsampling with Cartesian ordering) (20), a small-field-of-view fast spin echo T2-weighted sequence, a diffusion-weighted sequence (b = 0 and 500), and a delayed axial postgadolinium T1-weighted spoiled gradient echo sequence (LAVA Flex; GE Healthcare). In the abdomen bed position, the same sequences were acquired except for the dynamic contrast-enhanced sequence. For the whole-body acquisition, PET data were acquired for 3 min at each bed position with axial LAVA Flex and variable refocusing flip-angle single-shot fast spin echo sequences in the coronal and axial planes (21). Attenuation was corrected using a standard 2-point Dixon acquisition converted into an attenuation map as previously described (22).
Image Analysis
All 68Ga-PSMA-11 PET studies were interpreted and reported by a nuclear medicine physician and a radiologist masked to the clinicians’ pre- and postimaging treatment decisions. All PET images and cross-sectional images were available at the time of review. PET data were interpreted using an Advantage Workstation (version 5.0; GE Healthcare). Lesions were characterized as positive if they demonstrated uptake above the adjacent background level and if that uptake could not be attributed to physiologic biodistribution (e.g., urinary activity). Lesion location was categorized on the basis of the imaging report as prostate bed, pelvic lymph nodes, extrapelvic retroperitoneal nodes, other lymph nodes, osseous lesions, or visceral lesions.
Surveys and Analysis
The ordering team was asked to fill out a preimaging intended-treatment form and a postimaging intended-treatment form using methodology similar to that previously reported for various tumor types (23). On both surveys, clinicians were asked to categorize their intended management as surgery, RT, androgen-deprivation therapy, second-generation androgen receptor–targeted therapy (abiraterone or enzalutamide), active surveillance, biopsy, a modification of existing therapy, chemotherapy, radionuclide therapy (223Ra), or other. Additionally, they were asked to categorize the location of the patient’s disease as unknown, prostate bed, pelvic nodes, extrapelvic nodes, soft tissue, or bone. The preimaging survey also asked what test would have been ordered if 68Ga-PSMA-11 PET were not available, including MRI, CT, 18F-FDG or choline PET, bone scanning, ProstaScint, and image-guided biopsy. On the postimaging survey, the clinicians were asked if the ordering of a test had been prevented; they also were asked to list which test had not been ordered because of the 68Ga-PSMA-11 PET results.
Change in management was based on survey results and was categorized as major, minor, no change, or unknown on the basis of a predetermined categorization schema (supplemental material; supplemental materials are available at http://jnm.snmjournals.org). When clinicians checked “other” without clarifying the intended management, individual patient charts were reviewed by a genitourinary medical oncologist not involved in the care of the patient, and the patients were recategorized if chart review made clear the intended or implemented change. Biopsy was considered a form of active surveillance for our analysis. A χ2 test was used to compare the rate of major changes in patients treated with RP versus those treated with RT or with RT and RP.
RESULTS
A total of 150 patients with BCR were enrolled in this study, and both preimaging and postimaging intended-treatment surveys were received for 126 patients (survey response rate of 84%) (Table 1). The average PSA at the time of imaging was 5.9 ± 10.3 ng/dL, with 49 patients having a PSA of less than 2.0 at the time of imaging. In patients who were previously treated with RP, the average PSA was 2.7 ± 4.0 ng/dL; in those previously treated with RT, the average PSA was 9.9 ± 14.6 ng/dL; in those treated previously with both RP and RT, the average PSA was 3.9 ± 6.9 ng/dL.
On the preimaging survey, the most common imaging study that would have been ordered in place of 68Ga-PSMA-11 PET was 99mTc-labeled phosphate bone scanning in 70 (56%) of the patients (Table 2). On postimaging surveys, it was reported that studies were prevented from being ordered in 48 patients (38%). The most common prevented study was bone scanning in 21 patients (17%).
Imaging Studies That Would Have Been Ordered in Place of 68Ga-PSMA-11 PET on Preimaging Surveys, and Studies That Were Prevented from Being Ordered on Postimaging Surveys
Imaging Results
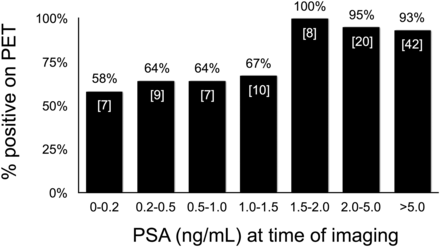
For 103 patients (82%), disease was detected on 68Ga-PSMA-11 PET at the time of imaging. 68Ga-PSMA-11 PET had a detection rate of above 50% at all PSA levels, including patients with a PSA of less than 0.2 ng/dL (Fig. 1). There was an inflection point at PSA values of 1.5 ng/dL or higher, at which the positive scan rate was 93% or higher. Categorized by PSA doubling time, detection rates were 83% (24/29), 90% (27/30), 97% (33/34), and 88% (21/24) for PSA doubling times of 0–3 mo, 3–6 mo, 6–12 mo, and greater than 12 mo. The 2 most common sites of disease on 68Ga-PSMA-11 PET were the prostate bed and pelvic lymph nodes, seen in 36% and 42% of patients, respectively (Fig. 2).
Percentage of patients with disease detected on 68Ga-PSMA-11 PET categorized by PSA level at time of imaging. Numbers in brackets are patients in each group with PET findings positive for disease. Percentage is percentage of patients in each group positive for disease.
Distribution of sites of disease seen on 68Ga-PSMA-11 PET as percentage of total patients. The most common sites of disease were prostate bed and pelvic lymph nodes (LN). RP = extrapelvic retroperitoneal.
68Ga-PSMA-11 PET decreased the percentage of patients with unknown sites of disease from 52% to 20% (Fig. 3). There was not perfect concordance between the reported sites of disease based on the clinical interpretation of the imaging study and the physician’s description of where the disease was thought to be. For example, clinicians reported pelvic nodes in 30% of patients after 68Ga-PSMA-11 PET, but the clinical reports described pelvic nodes in 42% of patients (Figs. 2 and 3).
Change in clinician’s description of disease location before and after imaging. Percentage of patients for whom clinicians did not know disease location decreased from 52% to 20%. ST = soft tissues.
Intended Management Results
There were 67 patients (53.2%) with major changes and 8 patients (6.4%) with minor changes in intended treatments (Table 3). The most common treatment change was a conversion to focal (targeted) treatment from systemic therapy, including 40 patients (31.7%) who received RT when a systemic therapy or active surveillance was initially planned (Fig. 4). Fifteen patients initially had unknown changes in management (“other” was selected on the survey form), which were converted to 1 major change, 1 minor change, 6 no changes, and 7 unknowns after chart review.
Changes in Intended Management After 68Ga-PSMA-11 PET
Example of major change in management. A 69-y-old man with biochemically recurrent prostate cancer was originally treated with RP in 2014 and then with salvage RT in 2015. He presented for 68Ga-PSMA-11 PET with PSA of 0.059. Imaging demonstrated single PSMA-positive lesion in right iliac bone (C), with no correlate seen on CT (A and [fused image] B). Management was converted from active surveillance to RT combined with androgen-deprivation therapy.
The percentage of major changes in management was relatively consistent across PSA levels at presentation. The percentage of patients with major changes in intended management with PSA levels of 0–0.2, 0.2–1.0, 1.0–2.0, 2.0–5.0, and greater than 5.0 ng/dL were 42%, 40%, 65%, 57%, and 56%, respectively. The percentage of patients with major changes in management did depend on prior treatment, with patients previously treated with RP having a lower rate than those treated with RT or with RP and RT (Table 4: RP vs. RT, P = 0.018; RP vs. RP and RT, P = 0.001; Table 4). Additionally, the percentage of patients with RT selected as the treatment on the preimaging survey was higher in patients previously treated with RP than in patients previously treated with RT (Table 4).
Patients with Major Changes in Management Categorized by Prior Treatment and PSA Level
DISCUSSION
68Ga-PSMA-11 PET scanning resulted in a major change in management in 53% of prostate cancer patients with BCR after definitive local therapy. A change from planned systemic therapy to focal targeted therapy such as RT was the most common change in management, occurring in 32% of patients. These results indicate that 68Ga-PSMA-11 PET plays an important role in the staging and management of men with prostate cancer in whom initial therapy fails. The results of this approach are currently being validated in a prospective multicenter trial.
Our results are consistent with prior reports on the impact of 68Ga-PSMA-11 on clinical management. Albisinni et al., in retrospectively reviewing 131 patients who underwent 68Ga-PSMA-11 PET, demonstrated a change in management in 75% of patients (15). Morigi et al. prospectively compared fluorocholine and 68Ga-PSMA-11, performed a retrospective survey of treating clinicians about how 68Ga-PSMA-11 PET changed management, and demonstrated a change in management in 63% of cases (17). Sterzing et al. retrospectively reviewed patients imaged before RT, evaluated the change on RT, and demonstrated a change in management in 51% of patients (16).
Our results showed that there was a lower level of change in management in patients after RP than in those treated with RT previously. This difference is likely caused by the fact that the standard therapy for RP patients is prostate-bed–only RT, as supported by the finding that 61% of post-RP patients had RT selected as the preimaging treatment selection. Because we did not evaluate changes in radiation field, disease outside the prostate bed that could not be targeted by radiation had to be demonstrated in order to show a major change in management in the post-RP population. The fact that we did not look at changes in radiation field and had a low change in management in post-RP patients is consistent with the results of Sterzing et al., who showed a high change in management in patients undergoing RT (51%), but only 7% of their patients were converted from RT to a different treatment modality (16).
The detection rate as a function of PSA level in this study agreed with previously published data (11,14). However, of 12 patients imaged with a PSA of less than 0.2 ng/dL, metastatic disease was detected in 7, suggesting that 68Ga-PSMA-11 PET may play a role in such patients. As confirmed in head-to-head comparisons, detection sensitivities in patients with 68Ga-PSMA-11 are significantly higher than shown with fluorocholine (17,24).
One major concern with 68Ga-PSMA-11 currently is that there is no understanding of how to use the added information provided by scanning to inform clinical decisions. In a large percentage of patients in this study, the therapy was converted to targeted RT because of oligometastatic disease seen on 68Ga-PSMA-11. However, a major limitation of this study is that it was not designed to evaluate whether this change in management resulted in improved outcomes. The potential benefit derived from improved imaging will require prospective testing that evaluates overall or progression-free survival as an endpoint. Although randomized prospective trials will not be required for Food and Drug Administration approval, they will be critical in obtaining insurance coverage in the future.
A second limitation of this study is that it did not prospectively collect information on changes in the planned radiation field in patients for whom RT was already planned. A potential major benefit of PSMA-11 PET is to provide information on which radiation fields will include all sites of disease (25). Sterzing et al. showed that 44% (25/57) of patients undergoing RT had a change in the radiation field that was used (16), suggesting that our results underestimated the change in management using PSMA-11 PET.
A third limitation is that not all patients received furosemide and that, therefore, there may be a limited detection rate for local recurrence in the 40 patients imaged without furosemide.
A fourth limitation is that the definition of BCR was based on PSA doubling time instead of on better-accepted criteria; a follow-up study is being performed using standard definitions of BCR.
A fifth limitation is the fact that the patients had varying conventional imaging studies performed, potentially affecting the preimaging intended management. In addition to the varying preimaging studies, the patients also underwent either PET/MRI or PET/CT, which provide different cross-sectional imaging correlates that might have affected the individual reads.
Finally, one of the inherent limitations of an analysis of change in intended management is the subjectivity in the interpretation of scan results by different providers and the bias that clinicians may have toward one particular treatment modality. Nevertheless, the current study did capture the full spectrum of clinical specialists who order PSMA-11 PET scans, namely urologists, radiation oncologists, and medical oncologists, and accurately reflects real-world clinical practice.
CONCLUSION
Existing treatment recommendations are based on staging using conventional imaging. 68Ga-PSMA-11 PET has a high detection rate that resulted in a major change in management in 53% of patients with BCR in our study. Further work should be performed to determine whether these changes in management result in improved outcome for patients.
DISCLOSURE
Thomas A. Hope receives grant support from GE Healthcare and is supported by the Radiological Society of North America and the Department of Radiology and Biomedical Imaging, UCSF. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online May 18, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 26, 2017.
- Accepted for publication May 9, 2017.