Abstract
68Ga-prostate-specific membrane antigen PET/CT (68Ga-PSMA PET/CT) offers unprecedented accuracy for staging of primary, persistent, or recurrent prostate cancer. Thus, we hypothesized that 68Ga-PSMA PET/CT before radiotherapy significantly affects the radiotherapeutic approach in comparison to the current standard, a CT-based approach. Methods: Between February 2014 and December 2017, 172 patients underwent 68Ga-PSMA PET/CT before radiotherapy and were included in this retrospective analysis. Twenty-two (13%) patients were referred for primary definitive radiotherapy, 51% (88/172) for prostate-specific antigen (PSA) persistence, and 36% (62/172) for PSA recurrence after radical prostatectomy. An experienced radiation oncologist, masked to the CT and PET/CT results, decided on the radiation treatment management of all patients on the basis of the clinical and pathologic variables. The potential increase in diagnostic accuracy, and the subsequent change in radiotherapeutic approach, were documented separately for PET/CT versus CT. Results: The overall detection rate was 70% (120/172) for 68Ga-PSMA PET/CT. Patients with a pre-PET/CT PSA level of more than 0.5 ng/mL (98/111; 88%) had PET-positive results significantly more often. Overall, PSMA PET/CT revealed 171 lesions, PET alone 156, and CT alone 85. For all patients, a continuous diagnostic increase in positive findings was observed for primary tumor/local recurrence (CT, 18%, vs. PET/CT, 37%), pelvic lymph nodes (CT, 21%, vs. PET/CT, 44%), and distant metastases (CT, 7%, vs. PET/CT, 19%) when comparing CT with PET/CT. Compared with CT, the combination of PET/CT information resulted in a change in treatment in 107 of 172 (62%) patients, that is, 8 of 22 (36%) patients before any treatment, 31 of 62 (50%) with PSA recurrence, and 68 of 88 (77%) with PSA persistence. Comparing the different radiotherapy indications with one another, there was a higher rate of change in management for postoperative patients than for patients before any treatment. Conclusion: Compared with conventional CT, 68Ga-PSMA PET/CT had a remarkable impact on radiotherapeutic approach, especially in postoperative patients. Thus, considering the growing amount of data on the impact of 68Ga-PSMA PET/CT on postoperative patients, 68Ga-PSMA PET/CT has recently been endorsed by a few cancer guidelines as an imaging modality in patients with PSA persistence or recurrence (e.g., the German S3 guideline and the European Association of Urology guideline).
Radiotherapy is a well-established standard approach for the curative treatment of prostate cancer. In the primary setting, both radical prostatectomy and radiotherapy with or without androgen deprivation therapy are viable options for patients with localized prostate cancer and have a similar oncologic outcome (1). So far, CT or MRI of the abdomen and pelvis and bone scintigraphy have been the standard of care in staging newly diagnosed prostate cancer (2). Likewise, radiotherapy plays a pivotal role in achieving tumor control in patients with persistent or recurrent prostate-specific antigen (PSA) after radical prostatectomy (3). The current guidelines of the European Association of Urology, European Society for Radiotherapy and Oncology, and International Society of Geriatric Oncology (4) concede that the diagnostic yield of common imaging techniques in staging prostate cancer after radical prostatectomy is poor and refer to MRI and choline PET/CT as possible imaging methods in patients with a PSA level of more than 1 ng/mL. Lately, 18F-fluciclovine (Axumin; Blue Earth Diagnostics) PET/CT or PET/MRI has been added to the clinical practice guidelines of the National Comprehensive Cancer Network on oncology for prostate cancer (version 1.2018) and is advised to be considered in the clinical workup of patients with recurrent prostate cancer. In the primary and postoperative settings, dose escalation to the primary tumor (5,6), local residua, or recurrences within the prostatic fossa (7–9) and involved lymph nodes possibly correlates with a better oncologic outcome. Consequently, an accurate detection of the individual prostate cancer distribution is mandatory to select suitable patients for individualized radiotherapy dose escalation within the pelvis. So far, standard target volumes and radiotherapy planning have been based on CT and MRI.
Lately, 68Ga-prostate-specific membrane antigen (68Ga-PSMA) PET/CT has emerged as the imaging modality with the highest sensitivity and specificity in staging prostate cancer, particularly with biochemical persistence or recurrence after radical prostatectomy, compared with conventional imaging such as CT or MRI (10,11) and choline PET/CT (12). Unlike conventional imaging, 68Ga-PSMA PET offers the possibility of visualizing prostate cancer residual disease or recurrence even at low PSA levels, with 58.3% of PET-positive results found in a PSA range of 0.51–1.0 ng/mL (13–18). Therefore, 68Ga-PSMA PET/CT might further improve oncologic outcome by modifying target volume delineation and intended overall doses. There is increasing evidence that 68Ga-PSMA PET/CT might have a major impact on radiotherapy planning in the primary (19–21) and postoperative settings (21–30).
As a standard operating procedure at our institution, evidence of PET-positive pelvic or paraaortic lymph nodes or PET-positive osseous oligometastases in patients scheduled for primary treatment of prostate cancer results in a change in radiotherapeutic approach. It triggers enlargement or expansion of pelvic volumes to include PSMA-positive pelvic nodal disease, or adjacent paraaortic disease, with an integrated or sequential boost. Bone metastases are treated with metastasis-directed radiotherapy, normally stereotactic body radiotherapy. In postoperative patients, local macroscopic tumor residua or recurrences lead to simultaneously integrated or sequential boost volumes to the local macroscopic tumor. Likewise, PET-positive pelvic or paraaortic lymph node metastases or a limited number of bone metastases in the sense of oligometastatic disease result in an adaptation of the irradiation volume with simultaneously integrated or sequential boosts (31,32). In the case of polymetastatic or visceral metastatic (M1c) disease, the treatment recommendation is primary androgen deprivation therapy or systemic therapy.
Performing 68Ga-PSMA PET/CT on a regular basis, we aimed in this retrospective analysis to assess the diagnostic value of 68Ga-PSMA PET/CT in differentiating persistent from recurrent PSA in treatment-naïve prostate cancer and postoperative patients and to evaluate the potential impact on radiotherapy planning.
MATERIALS AND METHODS
Study Population
Between February 2014 and December 2017, 1,492 patients with prostate cancer underwent 68Ga-PSMA PET/CT at the Department of Nuclear Medicine. Of this cohort, 8.7% (172/1,492 patients) underwent 68Ga-PSMA PET/CT before radiotherapy after referral to the Department of Radiation Oncology and were included in this retrospective analysis. Thirteen percent (22/172) of patients were referred for primary definitive radiotherapy, 51% (88/172) for PSA persistence, and 36% (62/172) for PSA recurrence after radical prostatectomy. Patients were subgrouped according to the D’Amico criteria (33) incorporating tumor stage, PSA level, and Gleason score (Table 1). All patients provided written informed consent to undergo 68Ga-PSMA PET/CT. This retrospective analysis was performed (with irreversibly anonymized patient data) in compliance with the principles of the Declaration of Helsinki and its subsequent amendments (34) and was approved by the local Ethics Committee (approval 556-16). The requirement to obtain informed consent was waived (per our local Ethics committee [Medical Faculty of the LMU Munich] written informed consent can be waived for irreversibly anonymized patient data).
Patients’ Characteristics
68Ga-PSMA Labeling and PET/CT Imaging
PSMA-HBED-CC was radiolabeled with 68Ga3+ from a 68Ge/68Ga generator system (GalliaPharm; Eckert and Ziegler AG) using an automated synthesis module (GRP; Scintomics GmbH) and prepacked cassettes (ABX GmbH) as described previously by Weineisen et al. for a different PSMA ligand (35). 68Ga-PSMA PET/CT imaging was performed according to current guidelines (36) with a Siemens Biograph 64 or GE Healthcare Discovery 690 PET/CT camera. Phantom studies based on the National Electrical Manufacturers Association NU2-2001 standard were conducted to allow valid pooling of the results, and SUV conversion factors were calculated (37). 68Ga-PSMA PET/CT scans were performed with a diagnostic CT scan (reference mAs, 200–240; 120 kV) and intravenous injection of iodine-containing contrast agent (Ultravist 300 [Bayer Pharma AG] or Imeron 300 [Bracco, Konstanz]; 2.5 mL/s; in the portal venous phase) 60 min after intravenous administration of 68Ga-PSMA (median, 205 MBq; range, 87–293). In the absence of contraindications, 20 mg of furosemide were injected almost simultaneously with 68Ga-PSMA injection and patients were asked to empty their bladder to minimize residual activity in the urinary system. PET images were reconstructed with an axial 168 × 168 matrix based on the TrueX algorithm (3 iterations, 21 subsets; Biograph 64) or with the VUE Point FX algorithm (2 iterations, 36 subsets; Discovery 690).
Image Interpretation
68Ga-PSMA PET/CT images were interpreted by a consensus read of 2 nuclear medicine physicians and 2 radiologists and additionally evaluated by an independent observer with more than 5 y of experience in 68Ga-PSMA PET/CT reading. The location of each lesion was determined by CT. PET-positive lesions were identified by 68Ga-PSMA uptake visually above the background level beyond physiologic uptake. On CT imaging, asymmetric focal areas of masslike contrast enhancement in the peripheral prostate detected on venous phase contrast-enhanced CT imaging or tumor penetration of the prostatic capsule was considered a positive finding of primary prostate cancer. Positive nodes were defined by a short-axis diameter of at least 1 cm, loss of fatty hilum, or increased contrast enhancement on CT. Bone metastases were detected as suspected sclerotic lesions. On the basis of the 68Ga-PSMA PET/CT images and reports, stage according to PET or CT was documented in consensus. For this analysis, all PSMA PET/CT scans of the included patients were reanalyzed by collecting the number of suspected local lesions, pelvic or paraaortic lymph nodes, and bone metastases and comparing each imaging modality (CT vs. PET vs. PET/CT) with the others.
Radiotherapy Before and After PSMA PET/CT Information
An experienced radiation oncologist, initially masked to the CT and PET/CT imaging results, decided on the treatment management of all patients on the basis of the clinical and pathologic variables, such as PSA, Gleason score, and TNM stage, before the results of the CT and 68Ga-PSMA PET/CT were available. Also before the findings of CT and 68Ga-PSMA PET/CT were available, prostate cancer patients referred for primary radiotherapy were stratified according to the D’Amico risk group classification as being at low, intermediate, or high risk (33). Depending on a patient’s D’Amico risk group and the risk for pelvic lymph node involvement according to the Memorial Sloan Kettering Cancer Center Prostate Cancer Nomogram, low-risk patients are normally treated with radiotherapy of the prostate alone, intermediate-risk patients with radiotherapy of the prostate plus concomitant androgen deprivation therapy for up to 6 mo (38), and high-risk patients with radiotherapy of the prostate and the pelvic lymphatic pathways plus androgen deprivation therapy for up to 2 y (39,40). Total doses applied to the prostate gland range from a normofractionated 74 Gy in low-risk patients to 76–78 Gy in intermediate- and high-risk patients. Pelvic lymphatic pathways are generally treated at our department with 50.4 Gy in 1.8-Gy daily fractions. Patients with PSA persistence are normally treated with radiotherapy delivered to the prostatic fossa alone, with 66 Gy in 2-Gy fractions, and in cases of pathologic lymph nodes at the time of radical prostatectomy, with irradiation of the pelvic lymphatic pathways as well (41). Patients with PSA recurrence tending to have primarily local recurrence are generally treated with irradiation of the prostatic fossa only (42). Subsequently, the participating radiation oncologist was first unmasked with respect to the CT information and assessed the change by CT information compared with the standard radiotherapy target volume. Likewise, the change by PET/CT information compared with a target volume based solely on the clinical and pathologic variables was assessed. The change in radiotherapeutic approach regarding CT and PET/CT information was documented separately for each patient, as well as the potential increase in diagnostic accuracy when comparing CT, PET, and PET/CT.
Statistical Analysis
For statistical analysis, SPSS Statistics, version 25 (IBM), was used. Descriptive analysis was performed by calculating the mean, median, and range. Frequencies of CT-, PET-, and PET/CT-positive cases were compared using the McNemar test. Differences in the short-axis diameter of lymph node metastases detected on CT and PET/CT were compared using the t test. A P value of less than 0.05 was considered statistically significant. Logistic regression analysis was performed to identify the D’Amico risk group (low/intermediate vs. high risk), PSA level before 68Ga-PSMA PET/CT (<0.5 vs. ≥0.5 ng/mL), primary versus postoperative status, and Gleason score (≤7b vs. >7b) as potential predictors for change in management.
RESULTS
68Ga-PSMA PET/CT Findings
One hundred twenty patients (120/172; 70%) showed at least 1 suspected lesion on 68Ga-PSMA PET/CT (Table 1). The median PSA level (ng/mL) in these patients before 68Ga-PSMA PET/CT was 1.9 (range, 0.14–40.13) in patients with PSA persistence (77%; 68/88), 0.78 (range, 0.27–6.24) in patients with PSA recurrence (50%; 31/62), and 13.7 (range, 0.14–150) in patients before definitive radiotherapy (95%; 21/22). Fifty-two patients (52/172; 30%) had no PET-positive findings on 68Ga-PSMA PET/CT. Among these patients, the median PSA level was 0.34 (range, 0.13–1.33) in those with PSA persistence (23%; 20/88), 0.3 (range, 0.15–3.24) in those with PSA recurrence (50%; 31/62), and 0.52 in 1 patient before definitive radiotherapy. Androgen deprivation therapy was ongoing in 19 patients (12 of the patients with persistent PSA, 3 with recurrent PSA, and 4 of the treatment-naïve patients) before 68Ga-PSMA PET/CT. Eighty-four percent of all patients (146/172) and 87% (104/120) of PET-positive patients were at high risk according to the D’Amico classification. In the PET-positive cohort, patients with PSA persistence accounted for most patients (57%; 68/120), whereas the PET-negative cohort primarily included patients with PSA recurrence (59%; 31/52).
An overview of findings according to CT versus PET versus PET/CT is presented in Table 2. Overall, PET/CT revealed 171 positive tumor lesions, PET alone revealed 156, and CT alone, 85. A continuous diagnostic increase in positive findings was observable for primary tumor/local recurrence (18% [31/172] for CT vs. 34% [58/172] for PET vs. 37% [63/172] for PET/CT), lymph node metastases (21% [36/172] for CT vs. 41% [71/172] for PET vs. 44% [76/172] for PET/CT), and distant metastases including nonregional lymph nodes (8% [13/172] for CT vs. 16% [28/172] for PET vs. 19% [32/172] for PET/CT) when comparing CT, PET, and PET/CT, with PET having significant superiority over CT alone (P < 0.05). Compared with CT, but not PET alone, PET/CT identified a significantly higher number of positive primary tumor/local recurrences, lymph nodes, and distant metastases. This observation was equally true for patients with definitive or postoperative radiotherapy with persistent or recurrent PSA.
Differences in TNM Staging with Regard to CT vs. PET vs. PET/CT
In Table 3, these data are specified for the detection and localization of lymph node metastases comparing CT with 68Ga-PSMA PET/CT: the detection rate of suggestive lymph nodes was significantly higher on PET/CT than on CT alone (289 vs. 85 lymph nodes, P < 0.02). The mean short-axis diameter of the smallest lymph node metastases detected on 68Ga-PSMA PET/CT was significantly smaller on PET/CT than on CT alone (5.8 vs. 9.9 mm, P < 0.001).
Number and Region of Suggestive Lymph Nodes on CT Compared with PET/CT
Impact of 68Ga-PSMA PET Imaging on Change in Management
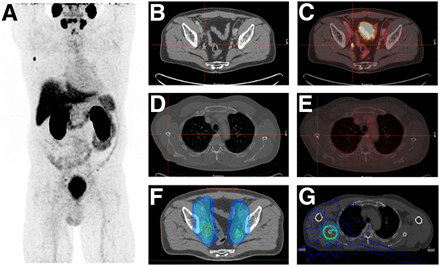
On the basis of the above-mentioned standard operating procedure criteria, 65% (112/172) of the cohort would have been treated before 68Ga-PSMA PET/CT with irradiation of the prostate/prostatic fossa alone and 35% (60/172) with radiotherapy of the prostate/prostatic fossa and lymphatic pathways (Table 4). CT information led to no change in treatment in 60% of patients (104/172) and to intensification in 40% (68/172). In 38% of patients (65/172), PET/CT information resulted in no change in treatment, and in 62% (107/172), PET/CT led to an intensification of treatment (e.g., enlargement of the radiotherapy volume because of irradiation of lymphatic pathways with or without simultaneously integrated or sequential boosts to macroscopic local residua or recurrences, suspected lymph nodes, or bone metastases). An example of a change in treatment according to CT and PET/CT information is shown in Figure 1 with the realized radiotherapy plan after 68Ga-PSMA PET/CT.
Changes in RT Protocol Due to CT and PET Information
A 59-y-old patient with Gleason 4 + 4 = 8 prostate cancer undergoing radiotherapy because of persistent PSA after radical prostatectomy. (A) Maximum-intensity projection shows local residual disease (covered by urinary bladder), single lymphatic, and single bone metastasis with high PSMA uptake. (B and C) Right iliac lymph node metastasis with malignant PSMA uptake on PET/CT (C) was not suggestive on CT (B) with just 3-mm short-axis diameter. (D and E) Single bone metastasis in lateral border of right scapula shows high uptake on PSMA PET/CT (E) but no correlate on CT (D). (F and G) Postoperative radiotherapy of former prostate gland (70 Gy) and pelvic lymphatic pathways (50.4 Gy) was performed, with simultaneous integrated boost to PET-positive iliac lymph node metastasis (56 Gy) (dose distribution in F). Further, stereotactic body radiotherapy (G) was given to single bone metastasis in right scapula (30 Gy).
Comparing the different radiotherapy indications with one another, 68Ga-PSMA PET/CT versus CT led to a higher incidence of change in management in postoperative patients than in patients with a definitive radiotherapy indication: compared with CT, 68Ga-PSMA PET/CT intensified the radiotherapeutic approach in 50% (31/62) versus 24% (15/62) of patients with PSA recurrence and in 77% (68/88) versus 53% (47/88) of patients with a radiotherapy indication due to PSA persistence. In patients with a definitive radiotherapy indication, CT led to a change in management in 27% (6/22) and 68Ga-PSMA PET/CT in 36% (8/22). Table 4 gives the absolute numbers of the respective radiotherapy indications for CT and PET/CT versus standard radiotherapy target volume definition.
Factors Predicting 68Ga-PSMA PET/CT–Based Change in Management
In the multivariate binary logistic regression analysis (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org), postoperative patients with either biochemical recurrence or persistence had a change in management significantly more often than patients with definitive radiotherapy. Likewise, a Gleason score higher than 7b or a PSA level before 68Ga-PSMA PET/CT of at least 0.5 ng/mL was significantly associated with a change in management. D’Amico risk group had no significant impact on change in treatment.
DISCUSSION
Recently, we reported on the clinical outcome of prostate cancer patients after 68Ga-PSMA PET/CT–based radiotherapy (8,9). The main aim of the present study was to assess the impact of 68Ga-PSMA PET/CT in the clinical setting and whether its high detection rate compared with CT alone translates into a substantial change in management in a heterogeneous group of prostate cancer patients referred for either definitive or postoperative radiotherapy. 68Ga-PSMA PET/CT was performed on 172 patients and demonstrated at least 1 suspected lesion in 70% (120/172) of patients.
Most studies available on the diagnostic performance of 68Ga-PSMA PET/CT analyzed patients with recurrent prostate cancer: detection rates range from 50% in patients with PSA levels of less than 0.5 ng/mL up to 73% in patients with PSA levels of 0.51–1.0 ng/mL (13–18). In the present analysis, 68Ga-PSMA PET/CT was negative in 30% (52/172) of patients. Considering the relatively low median PSA level in this subgroup and the fact that this subgroup consisted primarily of patients with biochemical recurrence, with a known tendency to relapse within the prostatic fossa, overshadowed by the SUV and radioactivity concentration within the bladder (43), the percentage of patients with a negative 68Ga-PSMA PET/CT result is plausible. Of all patients with negative PET results, there was only 1 patient with an indication for primary radiotherapy.
Indeed, the implementation of 68Ga-PSMA PET/CT for staging at initial diagnosis before radical prostatectomy or definitive radiotherapy is controversial at present and is not advised in current prostate cancer guidelines (2,44). However, compared with conventional imaging (CT, MRI) and bone scanning, several groups demonstrated the superiority of 68Ga-PSMA PET/CT in lymph node and bone metastasis staging (10,45,46). Overall, 68Ga-PSMA PET/CT was positive in 95% of patients with an indication for definitive radiotherapy in our analysis. Although patients before definitive radiotherapy constituted the smallest subgroup of the present cohort, there was a modest change in management (36%) compared with postoperative patients. This finding is in accordance with the few existing analyses on 68Ga-PSMA PET/CT in therapy-naïve patients before radiotherapy (19–21). In a recent analysis by Calais et al. based on 73 patients with localized untreated prostate cancer, a major impact of 68Ga-PSMA PET/CT was noted for 16.5% (12/73) of patients with intended irradiation of prostate, seminal vesicles, and pelvic lymphatic pathways and 37% of patients when radiotherapy fields covered prostate and seminal vesicles only (19). Likewise, Koerber et al. presented data on the impact of 68Ga-PSMA PET/CT on radiotherapy planning in 50 otherwise untreated prostate cancer patients (21). Similar to our analysis, they compared conventional imaging to 68Ga-PSMA PET/CT and saw an overall increase in lymph node metastases (10% vs. 16%) and distant metastases (6% vs. 10%). An increase in diagnostic yield was equally observed in our analysis when comparing CT to 68Ga-PSMA PET/CT regarding lymph node (18% vs. 41%) and distant metastases (5% vs. 14%). In total, 68Ga-PSMA PET/CT resulted in a change in radiotherapeutic management in 36% of our patients versus 44% of treatment-naïve patients included in the analysis by Koerber et al. Overall, the low number of patients intended for definitive radiotherapy is a limitation and needs further validation.
Contrary to the paucity of data on therapy-naïve patients, there is growing evidence on the superiority and high impact of 68Ga-PSMA PET/CT versus conventional imaging in staging patients with biochemical persistence or recurrence (21–30): in the present analysis, 68Ga-PSMA PET/CT detected, in total, 140 cases of residual or recurrent disease, lymph nodes, and distant metastases, whereas CT detected 64 lesions in postoperative patients.
Overall, there were 16% and 10% of patients with, respectively, persistent and recurrent PSA with evidence of local recurrence in the prostatic fossa triggering a dose escalation to the macroscopic tumor. This finding mirrors data by Habl et al. analyzing a high-risk group with biochemical failure after radical prostatectomy and observing local tumor recurrence on 68Ga-PSMA PET/CT versus conventional imaging in 28% of patients (47). Likewise, Bluemel et al. evaluated the diagnostic performance of 68Ga-PSMA PET compared with CT in a smaller number of postoperative patients with elevated PSA levels with regard to local recurrence (27): in 1 patient (9%) only CT was positive, whereas in 10 patients (91%) combined PET/CT was positive and in 5 patients (45.5%) only PET was positive.
Compared with CT, 68Ga-PSMA PET/CT was superior in the detection of pelvic lymph node metastases: among patients with recurrent and persistent PSA, there was evidence of positive pelvic lymph nodes in 23% (14/62) and 60% (53/88) on 68Ga-PSMA PET/CT versus 11% (7/62) and 28% (25/88) on CT only. Overall, 68Ga-PSMA PET/CT, compared with CT, resulted in an upstaging of 23% (35/150) of patients with biochemical recurrence and persistence. In a similar but smaller analysis by Sterzing et al., 68Ga-PSMA PET/CT upstaged 52% (15/29) of postoperative patients from N0 to N1 (30). In the combined analysis on 68Ga-PSMA PET/CT in postoperative patients with persistent or recurrent PSA by Koerber et al., a change in N-staging was observed in 28.2% (20/71) of patients, compared with conventional imaging (21).
So far, the present analysis is one of the few (22) specifically addressing patients with persistent and recurrent PSA separately. In our opinion, this is of high importance because patients with biochemical persistence are mostly a subgroup with a more advanced and aggressive tumor load with completely different metastatic progression patterns—that is, a high tendency to lymph node metastases, compared with patients with biochemical recurrence, who have mostly local recurrences in the prostatic fossa. Comparing the mere numbers of upstaged patients, the diagnostic yield of pelvic lymph nodes was 2.6 times higher in patients with biochemical persistence than in those with recurrence. Our departmental policy is that evidence of pelvic lymph nodes should lead to irradiation of the pelvic lymphatic basin according to the Radiation Therapy Oncology Group consensus recommendations on delineation of pelvic lymphatic pathways (40) with a simultaneous boost to the PET-positive lymph nodules and additional androgen deprivation therapy. Interestingly, there is strong controversy on the best therapeutic approach in cases of positive lymph nodes, with some centers opting for stereotactic body radiotherapy to the PET-positive lymph nodes only (48). Having recently presented our data on outcome in patients with biochemical recurrence treated on the basis of the results of 68Ga-PSMA PET/CT (9), we believe that eradicating microscopic spread to surrounding lymphatic pathways, dose escalation to macroscopic tumor burden, and at least concomitant use of androgen deprivation therapy might be more favorable than stereotactic body radiotherapy of PET-positive nodes only.
As expected, patients with biochemical recurrence had significantly fewer distant metastases on 68Ga-PSMA PET/CT (2/62 patients), as well as on CT (1/62 patients), than did patients with PSA persistence (27/88 on 68Ga-PSMA PET/CT and 11/88 on CT). Overall, a change in M-staging was present in 11% (17/150). This is lower than the change in M-staging (22.5%) that was observed in the study by Koerber et al. (21), most likely because the patients in their analysis had a significantly higher PSA before 68Ga-PSMA PET/CT (3.06 ng/mL) than did patients in the present analysis. PSA (obtained before PET/CT imaging), Gleason score, and resection status (primary tumor without prior surgery and status after surgery) had an impact on change in management. In binary logistic regression analysis, patients with prior surgery, a Gleason score higher than 7b, and a PSA higher than 0.5 had a higher probability for change in radiotherapy planning.
The present analysis has limitations due to its monocentric design, a possible referral bias of patients intended for radiotherapy, and an overall limited number of patients, especially before any treatment. Thus, a larger and multicenter analysis on the impact of 68Ga-PSMA PET/CT on change in therapeutic management could provide further clarification. For patients in whom salvage radiotherapy is indicated, there is a currently recruiting phase III trial randomizing patients to undergoing 68Ga-PSMA PET/CT, or not, before salvage radiotherapy (https://clinicaltrials.gov/ct2/show/NCT03582774) that will further clarify the high impact of 68Ga-PSMA PET/CT before radiotherapy in the postoperative setting.
CONCLUSION
Compared with conventional CT or an approach based on clinical factors only, 68Ga-PSMA PET/CT had a remarkable impact on the radiotherapeutic approach, especially in postoperative patients, with a consecutive intensification of treatment in 50% (31/62) of patients with recurrent PSA and 77% (68/88) of patients with persistent PSA. Thus, considering the growing amount of data on the impact of 68Ga-PSMA PET/CT on postoperative patients, 68Ga-PSMA PET/CT has recently been recommended as an imaging modality in patients with PSA persistence or recurrence in a few cancer guidelines, such as the European Association of Urology guideline and the German S3 guideline.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Dec. 14, 2018.
- © 2019 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 25, 2018.
- Accepted for publication November 28, 2018.