Visual Abstract
Abstract
Despite a high detection rate of 68Ga-prostate-specific membrane antigen (PSMA) PET/CT in biochemical recurrence (BCR) of prostate cancer, a significant proportion of men have negative 68Ga-PSMA-11 PET/CT results. Gastrin-releasing peptide receptor, targeted by the copper-chelated bombesin analog 64Cu-sarcophagine-bombesin (SAR-BBN) PET/CT, is also overexpressed in prostate cancer. In this prospective imaging study, we investigate the detection rate of 64Cu-SAR-BBN PET/CT in patients with BCR and negative or equivocal 68Ga-PSMA-11 PET/CT results. Methods: Men with confirmed adenocarcinoma of the prostate, prior definitive therapy, and BCR (defined as a prostate-specific antigen [PSA] level > 0.2 ng/mL) with negative or equivocal 68Ga-PSMA-11 PET/CT results within 3 mo were eligible for enrollment. 64Cu-SAR-BBN PET/CT scans were acquired at 1 and 3 h after administration of 200 MBq of 64Cu-SAR-BBN, with further delayed imaging undertaken optionally at 24 h. PSA (ng/mL) was determined at baseline. All PET (PSMA and bombesin) scans were assessed visually. Images were read with masking of the clinical results by 2 experienced nuclear medicine specialists, with a third reader in cases of discordance. Accuracy was defined using a standard of truth that included biopsy confirmation, confirmatory imaging, or response to targeted treatment. Results: Twenty-five patients were enrolled. Prior definitive therapy was radical prostatectomy (n = 24, 96%) or radiotherapy (n = 1, 4%). The median time since definitive therapy was 7 y (interquartile range [IQR], 4–11 y), and the Gleason score was 7 or less (n = 15, 60%), 8 (n = 3, 12%), or 9 (n = 7, 28%). The median PSA was 0.69 ng/mL (IQR, 0.28–2.45 ng/mL). Baseline PSMA PET scans were negative in 19 patients (76%) and equivocal in 6 (24%). 64Cu-SAR-BBN PET–avid disease was identified in 44% (11/25): 12% (3/25) with local recurrence, 20% (5/25) with pelvic node metastases, and 12% (3/25) with distant metastases. The κ-score between readers was 0.49 (95% CI, 0.16–0.82). Patients were followed up for a median of 10 mo (IQR, 9–12 mo). Bombesin PET/CT results were true-positive in 5 of 25 patients (20%), false-positive in 2 of 25 (8%), false-negative in 7 of 25 (28%), and unverified in 11 of 25 (44%). Conclusion: 64Cu-SAR-BBN PET/CT demonstrated sites of disease recurrence in 44% of BCR cases with negative or equivocal 68Ga-PSMA-11 PET/CT results. Further evaluation to confirm diagnostic benefit is warranted.
Despite curative-intent treatment, approximately 30% of men with prostate cancer will develop biochemical recurrence (BCR) as evidenced by a rising prostate-specific antigen (PSA) level (1,2). The ability to accurately detect sites of relapse in BCR is crucial to guide treatment recommendations, such as salvage radiotherapy, which may be curative in those with disease still confined to the prostate or pelvis. 68Ga-prostate-specific membrane antigen (PSMA) PET/CT is an effective imaging modality for detection of recurrent disease in men with BCR (3). However, a significant proportion of patients with BCR will have a negative PSMA PET/CT result even at higher PSA levels (4,5). Although some cases are likely due to the low volume of recurrent disease, a proportion may be because low PSMA expression on the individual’s cancer cells limits detection by PSMA PET/CT (6). Gastrin-releasing peptide receptor (GRPR) is overexpressed in some prostate adenocarcinomas, particularly in well-differentiated, lower-grade adenocarcinomas, with an inverse relationship between GRPR and PSMA expression (7). Bombesin is a 14-amino-acid amphibian homolog of the mammalian gastrin-releasing peptide, with a high affinity for GRPR, and its analogs have previously demonstrated promising safety and utility in the assessment of prostate adenocarcinoma (8,9). However, it has not been evaluated for additive clinical utility in those patients with BCR and negative or equivocal PSMA PET findings. We investigated the diagnostic value of 64Cu-sarcophagine-bombesin (SAR-BBN) PET/CT imaging in men with BCR after definitive primary treatment and negative or equivocal PSMA PET/CT results.
MATERIALS AND METHODS
Study Design
This prospective phase 2 imaging study was undertaken at a single Australian Institution (St. Vincent’s Hospital Sydney). The study protocol was approved by the St. Vincent’s Hospital institutional review board (HREC/2022/SVH/ETH00876), and patients provided informed and written consent. The study was registered with ClinicalTrials.gov (NCT0561384).
Patient Enrollment
Eligible participants had BCR prostate adenocarcinoma, defined as a PSA level rising by more than 0.2 ng/mL despite prior definitive treatment with radical prostatectomy or radiotherapy, along with negative or equivocal PSMA PET results within 3 mo of study enrollment. Patients with Eastern Cooperative Oncology Group performance status greater than 2, significant active cancers other than prostate cancer, currently on systemic anticancer treatments including androgen deprivation therapy (ADT), or with significant renal impairment (estimated glomerular filtration rate < 40 mL/min/1.73 m2) were excluded. Clinical information including prior prostate cancer treatments, histologic parameters at diagnosis (International Society of Urological Pathology grade group, presence of extracapsular extension, seminal vesicle involvement, lymph node involvement), historical PSA levels, and PSA doubling time was documented.
64Cu-SAR-BBN Production and Image Acquisition
64Cu-SAR-BBN was prepared by the South Australian Health and Medical Research Institute using a modified version of a previously described method (10). 64Cu-SAR-BBN (200 MBq) was injected intravenously within 24 h of production. PET imaging was performed at 1 and 3 h after injection and at an optional 24-h time point after injection. Images were acquired on a Siemens Biograph Vision 600-64 PET/CT scanner at a rate of 3 min over the first 2 field of views and 2 min over subsequent fields of view. Whole-body bombesin PET (skull vertex to mid thigh) was preceded by a noncontrast low-dose CT scan using a slice thickness of 2 mm with increment of 2 mm, soft-tissue reconstruction kernel, 120 kV and 50 mAs, pitch of 0.8, and matrix of 440. Emission data were corrected for randoms, scatter, and decay.
Imaging Analysis
64Cu-SAR-BBN PET/CT images were reported by 2 experienced nuclear medicine specialists who were masked to clinical data and prior imaging results. All images were reviewed and reported on fusion viewer software (syngo.via; Siemens). Images were analyzed visually, and lesions were reported on the basis of anatomic site, size, intensity of tracer uptake, and a 4-point scale of diagnostic certainty (definitely negative, equivocal–probably negative, equivocal–probably positive, and definitely positive). In cases of discordance between clinical reads, a third masked nuclear medicine specialist provided a tiebreaker opinion. Images were reported and made available to treating clinicians. Subsequent treatment was documented but left to the treating clinician.
Patient Follow-up
Patients were reviewed for adverse events after completion of each scan and 48 h after 64Cu-SAR-BBN administration. Data on investigations (PSA levels, histologic results, further confirmatory imaging) and the treatments initiated were collected.
Reference Standard for Accuracy
A previously published reference standard using a composite of histologic and PSA outcomes was used to determine scan accuracy (11). A true-positive finding was determined by either histologic confirmation (1/25) or PSA reduction after targeted therapy at a 64Cu-SAR-BBN–positive site of disease (4/25). A false-positive result was determined by an increase in PSA after targeted therapy (2/25). A false-negative result was determined by an increase in PSA without treatment or a reduction in PSA with targeted treatment in patients with a negative imaging result (7/25). A true-negative imaging result was a negative imaging result accompanied by a decrease in PSA without treatment. Accuracy could not be verified in patients who subsequently commenced ADT, had stable PSA levels, or were not followed up with documented PSA (11/25).
RESULTS
Patient and Imaging Characteristics
Twenty-five participants were enrolled, and their characteristics are detailed in Table 1; 96% (24/25) had prior radical prostatectomy, 4% (1/25) had brachytherapy, and 64% (16/25) had salvage radiotherapy. The median PSA at imaging with 64Cu-SAR-BBN was 0.69 ng/mL (interquartile range [IQR], 0.28–2.45 ng/mL). At enrollment, 76% (19/25) of participants had negative PSMA PET results and 24% (6/25) had equivocal PSMA PET results.
Patient Characteristics
64Cu-SAR-BBN imaging was performed at a median of 34 d (IQR, 21–77 d) after PSMA PET. 64Cu-SAR-BBN PET was performed at a median of 61 min (60–67) after 64Cu-SAR-BBN administration, and subsequent scans were obtained at 180 min (180–183). Sixty-four percent (16/25) of patients returned for optional delayed imaging, obtained at a median of 21 h (range, 20 h 57 min to 21 h 41 min). Patients were followed up for a median of 10 mo (IQR, 9–12 mo).
Detection Rate
64Cu-SAR-BBN–avid disease was identified in 44% (11/25) of participants. Of patients with positive 64Cu-SAR-BBN PET results, 37% (3/11) had prostate bed recurrence, 46% (5/11) had pelvic node disease, and 27% (3/11) had distant disease (lung and bone) (Figs. 1 and 2). 64Cu-SAR-BBN–avid lesions detected at the initial time point (1 h) were detected at all later time points except one—a time point at which a halo artifact from intense bladder uptake obscured a lesion at the right urethral anastomosis. Delayed imaging did not improve detection rate or certainty.
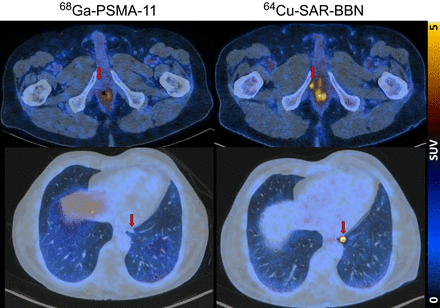
Fused PET/CT, maximum-intensity projection PET, and lung-windowed CT images (from left to right) from 64Cu-SAR-BBN (top row) and 68Ga PSMA-11 (bottom row) PET/CT study of patient demonstrating left subpleural lesion (arrows, SUVmax of 10 at 1 h) that showed 64Cu-SAR-BBN uptake but no 68Ga-PSMA-11 uptake. PSA was 1.84 ng/mL at time of imaging. This patient underwent lobectomy, with histopathology demonstrating metastatic prostate cancer.
Fused PET/CT, maximum-intensity projection PET, and CT images (from left to right) from 64Cu-SAR-BBN (top row) and 68Ga-PSMA-11 (bottom row) PET/CT study of patient demonstrating uptake at right urethral anastomosis (arrows, SUVmax of 5.2 at 1 h) on 64Cu-SAR-BBN alone. PSA was 2.5 ng/mL at time of imaging. This patient was managed with local radiotherapy alone, with improvement in PSA after treatment.
In patients with 64Cu-SAR-BBN–positive findings, the median PSA level was 1.33 ng/mL (IQR, 0.63–4.72 ng/mL) and the median doubling time was 4.0 mo (IQR, 3.2–7.3 mo). The median PSA level of those with negative 64Cu-SAR-BBN results was 0.74 ng/mL (IQR, 0.37–2.78 ng/mL), and the median doubling time was 4.4 mo (IQR, 2.4–8.8 mo) (Table 2).
Characteristics of Patients with Positive and Negative 64Cu-SAR-BBN Results
Reproducibility
The Cohen κ-score between the 2 primary readers was 0.49 (95% CI, 0.16–0.82), demonstrating moderate interrater reliability. 64Cu-SAR-BBN PET/CT images from 6 of 25 (24%) participants had discordant findings between the 2 reviewers and required a third tie-breaking review. These were low-volume local recurrences in 3 of 6, pelvic lymph nodes in 2 of 6, and a vertebral lesion in 1 of 6.
Diagnostic Accuracy
On the basis of the standard of truth, 64Cu-SAR-BBN PET/CT results were confirmed to be true-positive in 5 of 25 patients (20%), false-positive in 2 of 25 (8%), and false-negative in 7 of 25 (28%), whereas 11 of 25 (44%) could not have their results verified because of commencement of ADT (2/11), stable PSA (5/11), or lack of confirmatory results (4/11).
Treatment Outcomes
Of patients with positive 64Cu-SAR-BBN PET/CT results, 81% (9/11) underwent subsequent treatment. Salvage radiotherapy without ADT was performed on 2 patients with local disease. Targeted radiotherapy without ADT was performed on 3 patients with nodal disease and 1 patient with distant bone disease. ADT was commenced in 2 patients. One patient with bombesin PET–positive disease in the lung underwent a lobectomy.
Treatment plans were changed by positive 64Cu-SAR-BBN PET/CT results in 73% patients (8/11): 5 patients commenced or changed radiotherapy plans, 1 commenced ADT, 1 underwent a lobectomy, and 1 had a change from continued surveillance to planned biopsy (Table 3).
Treatment and Follow-up of Patients with Positive 64Cu-SAR-BBN PET Findings
Of patients with negative 64Cu-SAR-BBN PET/CT results, 7% (1/14) underwent further treatment (pelvic node radiotherapy), 43% (6/14) had persistent rising PSA levels, 36% (5/14) had stable PSA at follow-up, and 21% (3/14) were lost to follow-up. No patients demonstrated a PSA reduction.
No adverse events from the 64Cu-SAR-BBN PET/CT were reported at the 48-h safety assessment or by the treating physician.
DISCUSSION
PSMA PET has revolutionized patient management in BCR prostate cancer after curative-intent primary therapy (3,12). It is now approved for imaging of BCR in the National Comprehensive Cancer Network guidelines and in the Society of Nuclear Medicine and Molecular Imaging appropriate use criteria (13,14). However, negative PSMA PET results remain common at lower PSA levels at which patients are curable with salvage therapy (5). Additional diagnostic tools beyond PSMA PET that further stratify patients who have distant disease and who would not be cured by salvage local treatment would be clinically valuable. This study confirmed that targeting of alternative receptors to PSMA in prostate cancer may add diagnostic value and have an impact on management.
The GRPR is overexpressed in many prostate adenocarcinomas, particularly in well-differentiated, lower-grade adenocarcinomas, with an inverse relationship between GRPR and PSMA expression (15). 64Cu-SAR-BBN is a GRPR antagonist conjugated to a sarcophagine derivative and radiolabeled with 64Cu (a positron-emitting radioisotope). It has previously demonstrated high affinity for GRPR and high-contrast PET images in animal studies, as well as tumor binding with 64Cu-SAR-BBN PET/CT in hormone-positive breast cancer in a first-in-human study (10,16).
This study found that 64Cu-SAR-BBN PET/CT showed a detection rate of 44% in BCR with either negative or equivocal PSMA PET/CT results. The management impact was high in these patients, with 73% of patients having a documented change in management as a result of positive 64Cu-SAR-BBN results. These findings support the potential role of 64Cu-SAR-BBN PET/CT as a second-line imaging modality for BCR. Around half the patients in our cohort had negative 64Cu-SAR-BBN PET/CT results. This may be reflective of small-volume disease below the resolution limits of PET or of low-tumor GRPR expression.
64Cu is a positron-emitting radionuclide with a 12-h half-life. In a phantom-based analysis, 64Cu and 18F were found to have similar spatial resolution and image quality, both of which were superior to those of 68Ga (17). A longer half-life potentially improves detection of small-volume prostate fossa recurrences and dosimetry potential. However, delayed imaging at 3 and 24 h in this study did not increase detection rates with 64Cu-bombesin.
There was only moderate interrater reliability between our 2 primary readers. This likely reflects a lack of familiarity with a novel radiotracer, as the reviewers were not given a standard training sample to establish agreement before they performed their reviews. This issue would be expected to improve with increased use of this imaging radiotracer but requires further evaluation in future studies.
This study had several limitations, most notably that only a single 64Cu-SAR-BBN–avid lesion could be histologically confirmed. Because of the nature of the small volume of recurrent disease in the study, it was impractical to perform histologic sampling at many of the sites identified. A standard of truth that incorporates biopsy and response to targeted treatment allows only a limited estimation of accuracy. Although this is one of the larger studies into GRPR-targeting PET in BCR, the cohort remains small, and studies with higher numbers of patients are required to further assess both the diagnostic utility and the theranostics potential of this tracer. The small trial size also limits evaluation of the characteristics of patients with BCR who may best benefit from 64Cu-SAR-BBN PET/CT, although detection rates were higher at higher PSA levels. The promising results from this study suggest that larger trials may be warranted to further evaluate benefit.
CONCLUSION
64Cu-SAR-BBN PET/CT has the potential to detect sites of disease recurrence in BCR cases with negative or equivocal 68Ga-PSMA-11 PET/CT results. Further evaluation to confirm which patient subgroups would derive diagnostic benefit is warranted in larger studies.
DISCLOSURE
Louise Emmett reports receiving trial support from the PCF Challenge, Movember, Novartis, Clarity Pharma, and the St. Vincent’s Curran Foundation; being on an advisory board for Novartis, Advancell, Astellas, and Clarity Pharma; and being on a speakers’ bureau for Novartis, Astellas, Astrazeneca, Janssen, and Telix. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 64Cu-SAR-BBN PET/CT able to identify sites of disease in prostate cancer patients with BCR and negative or equivocal 68Ga-PSMA-11 PET/CT results?
PERTINENT FINDINGS: In this prospective phase 2 single-site imaging study involving 25 patients with BCR and negative or equivocal 68Ga-PSMA-11 PET/CT findings, 64Cu-SAR-BBN PET/CT identified sites of disease recurrence in 44% of patients. These findings were verified as true-positive disease in 5 of 11 patients.
IMPLICATIONS FOR PATIENT CARE: 64Cu-SAR-BBN PET/CT could identify further sites of distant disease in BCR of prostate cancer for targeted therapy.
Footnotes
Published online Aug. 1, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 3, 2024.
- Accepted for publication June 5, 2024.