Abstract
Dynamic bone scanning with 99mTc-labeled diphosphonates and 18F-labeled sodium fluoride provides functional information sensitive for subtle changes in bone turnover and perfusion, which assists the clinical management of numerous osseous pathologies. This article reviews the mechanisms of uptake of 99mTc-labeled diphosphonates and 18F-sodium fluoride and discusses and compares the performance of these bone-seeking radiotracers for clinical and research applications, using dynamic and multiple-time-point imaging protocols and quantitative techniques.
Sodium fluoride labeled with 18F (18F-NaF) for bone scanning was introduced in 1962 by Blau et al. (1) and subsequently was approved for clinical use by the U.S. Food and Drug Administration in 1972. At the time, images were acquired with rectilinear scanners equipped with thick NaI(Tl) crystals. After the introduction and widespread acceptance of new thin-crystal γ-cameras optimized for imaging with 99mTc, the introduction of 99mTc-labeled diphosphonates (99mTc-methyl diphosphonate [99mTc-MDP] and 99mTc-hydroxymethylene diphosphonate) replaced 18F-NaF bone scans in the mid 1970s.
The widespread availability of PET and PET/CT cameras in the United States has led to renewed interest in 18F-NaF bone scanning, with 18F-NaF PET first described in 1993 for clinical use (2). It provides highly sensitive, 3-dimensional imaging of the skeleton, with demonstrable utility in a growing range of benign and malignant bone disorders. 18F-NaF PET has the advantages of high spatial resolution, attenuation correction, 3-dimensional tomographic images, and hybrid PET/CT imaging (3–5). 18F-NaF PET is amenable to kinetic modeling, in particular quantitative assessment of bone perfusion and bone turnover, readily derived from dynamic PET acquisition, typically over 60 min (3–5). Recent guidelines have been published by the Society of Nuclear Medicine and Molecular Imaging for 18F-NaF PET and PET/CT (6).
UPTAKE MECHANISMS OF BONE-SEEKING RADIOPHARMACEUTICALS
Bone is a composite material of inorganic crystals bound to protein. The mineral phase, built of crystals containing mainly calcium and phosphate, is called hydroxyapatite. This mineral phase is bound to a matrix largely consisting of a single protein, collagen. The mineral content determines the stiffness of bone. Without sufficient mineralization, bones will plastically deform under load. Collagen provides toughness to bone, making it less brittle so that it better resists mechanical stress. The healthy adult bone is in homeostasis, with a constant rate of remodeling due to the activity of osteoblasts laying down new bone and osteoclasts resorbing old bone. Bone adapts to repetitive mechanical stresses largely by changing its size and shape, which are major determinants of its resistance to fracture (7).
Anatomically, bone tissue consists of compact (cortical) and cancellous (trabecular) bone, which perform different functions; compact bone forms the diaphyses of long bones and the surface of flat bones, whereas cancellous bone is found in the epiphyseal and metaphyseal regions of long bones and the interior of flat bones. Bone is in a constant state of remodeling or turnover, which is essentially a surface phenomenon. Although cortical bone forms most of the bone mass, it represents the minority of bone surface. By comparison, cancellous bone forms only 20% of the bone mass but accounts for 80% of the bone turnover associated with remodeling (8).
Physiologic Mechanism of Radiopharmaceutical Uptake
The pharmacokinetics of bone-seeking radiotracers essentially depend on the rates of bone uptake and elimination from the circulation via renal excretion.
Radiotracer Delivery
99mTc-labeled diphosphonates (99mTc-MDP and 99mTc-hydroxymethylenediphosphonate) and 18F-NaF are essentially markers of both bone perfusion and bone turnover (9). After intravenous administration, the principal uptake mechanism of bone-seeking radiotracers involves adsorption onto or into the crystalline structure of hydroxyapatite. The first step in this cascade is radiotracer delivery, which depends on local blood flow and the rate of radiotracer extraction by bone. 99mTc-MDP undergoes protein binding in blood, which increases over time from around 25% at injection to about 50% at 4 h after injection (10). Only unbound tracer will be available for bone uptake. Although 18F-NaF kinetics are not affected by protein binding, 18F-NaF is transported by red blood cells with an erythrocyte concentration of roughly half that in plasma. As a result, 18F-NaF concentrations are about 10%–25% higher in plasma than in whole blood (11).
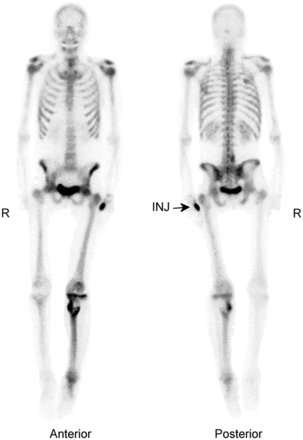
According to the simplified Renkin and Crone capillary transport model (12), the net exchange of a substance between blood and tissue depends on blood flow, surface area, and the permeability of the capillary system. 99mTc-MDP undergoes passive diffusion through the capillary wall into the extravascular space (13). The diffusion rate is proportional to the molecular size; therefore, diffusion of small molecules is expected to be more rapid than that of 99mTc-diphosphonates (14). In addition, there is evidence that the extraction fraction of 99mTc-diphosphonates varies with blood flow. Performing outflow dilution experiments in the tibia of dogs, McCarthy et al. showed that the extraction fraction of 99mTc-MDP decreased substantially with increasing blood flow (15). In addition, they determined that the permeability-surface area remained unchanged, indicating that additional recruitment of capillaries under high-flow conditions does not occur. Figure 1 demonstrates the effect of increased blood flow on 99mTc-MDP uptake to the entire lower extremity secondary to the presence of a primary bone malignancy.
Diffusely increased 99mTc-MDP uptake of left lower extremity on delayed-phase whole-body bone scan (anterior and posterior projections) as result of increased blood flow due to osteosarcoma in proximal tibia. INJ = injection site.
In contrast to γ-camera imaging, PET imaging allows absolute quantification of radiotracer concentrations in tissue. Dynamic PET compartment modeling is needed to measure bone blood flow and the metabolic rate of fluoride binding to bone. Using this technique, we previously measured bone blood flow and extraction fraction of 18F-NaF noninvasively, comparing the uptake of 15O-H2O and 18F-NaF in vertebral bodies of mini pigs (11). The mean vertebral blood flow (K1) as measured by 15O-H2O was 0.145 ± 0.047 mL/(min⋅cm3) using a 1-tissue-compartment model. Under isometric low-intensity exercise, bone blood flow increases 2.3-fold in humans (16). At typical flow rates in bone, 15O-H2O is a freely diffusible tracer; thus, its extraction into tissue approaches 100% in a single capillary passage (17). Since K1 is the product of blood flow and the extraction fraction, the quotient of corresponding 15O-H2O and K1 values is an estimate for the regional extraction fraction of 18F-NaF. Our results indicated that the extraction fraction of 18F-NaF decreased from about 100% at low-flow conditions to approximately 40% at high flow (11). Correcting K1 on the basis of extraction fraction estimates allows bone blood flow measurement with dynamic 18F-NaF PET (18). Since measurements of the extraction fraction for 18F-NaF and 99mTc-diphosphonates have been performed using different techniques, their results cannot be directly compared. However, it appears that the extraction fraction of 99mTc-diphosphonates is indeed substantially smaller than that of 18F-NaF.
Radiotracer Localization to Bone
The mechanism of binding of extravascular 99mTc-diphosphonates to bone is due to physicochemical adsorption (chemisorption) to the hydroxyapatite structure of bone tissue (19). Using autoradiography, the deposition of 99mTc-diphosphonates was found to occur at the mineralization front of bone (osteoid) and at the osteocytic lacunae, but not near osteoclasts (20). In the growing skeleton, mineral deposition is predominantly seen at epiphyseal growth plates and osteochondral junctions (21).
In contrast to 99mTc-diphosphonates, a certain fraction of extravascular fluoride is directly incorporated into the bone matrix, because fluoride ions exchange with hydroxyl groups in the hydroxyapatite crystal of bone to form fluoroapatite (22). One would therefore expect the net transport of fluoride into bone to be closely related to bone metabolism. In fact, dynamic 18F-NaF provides quantitative estimates of bone metabolism (via net transport constant) that correlate well with the mineral apposition rate obtained from bone histomorphometry (18,23), the most accurate method for the investigation of bone formation. Bone histomorphometry provides several static parameters that are important in assessing the microarchitecture of bone. The dynamic parameter that quantitatively reflects bone formation, the mineral apposition rate, requires double-tetracycline labeling of bone biopsy samples. In addition, bone histomorphometry is an invasive procedure requiring sedation and can sample only a single site of bone, usually the iliac crest. In clinical practice, bone histomorphometry is substituted with biochemical serum markers of bone metabolism, including alkaline phosphatase, and urinary N-telopeptide and C-telopeptide of collagen cross-links (3). Although such markers display relatively rapid treatment-induced changes, they are significantly limited by large day-to-day variations and insensitivity to regional changes because they reflect bone metabolism of the entire skeleton (24). Therefore, quantitative imaging of bone metabolism is of considerable interest for specialized clinical indications. The above data indicate that noninvasive dynamic 18F-NaF PET with determination of the net transport constant of 18F-NaF to bone is indeed a suitable surrogate marker of the mineral apposition rate.
Renal Excretion
99mTc-diphosphonate compounds belong to the class of bisphosphonates, which are not significantly metabolized in vivo (25). Thus, renal excretion is the primary route for their elimination. Hyldstrup et al. showed that the renal filtration fraction of free (non–protein-bound) 99mTc-MDP is the same as that for 51Cr-ethylenediamine tetraacetic acid, itself a measure of the glomerular filtration rate (26); thus, the renal elimination rate of 99mTc-diphosphonates depends mainly on glomerular function. Since the plasma binding of 18F-NaF is small, fluoride ions are freely filtered in the glomeruli. Fluoride, however, also undergoes tubular reabsorption. As a result, renal clearance of 18F-NaF is dependent on overall urinary flow, because tubular reabsorption of fluoride increases with decreasing glomerular filtration rate (3). Therefore, it is recommended that patients undergoing 18F-NaF PET be well hydrated to reduce radiation exposure.
Environmental Factors
Other environmental factors may influence the accumulation of 99mTc-MDP and 18F-NaF as well. Although renal clearance of 18F-NaF is modulated by urinary pH and diet, renal clearance of 99mTc-MDP is affected by phosphate concentration and pH (27). Also, the adsorption of 99mTc-MDP to hydroxyapatite appears to increase at low pH (28).
Quantification Methods
Short-lived radiotracers such as 99mTc-labeled diphosphonates and 18F-NaF allow for kinetic modeling, which provides simplified mathematic models to quantitatively describe aspects of bone perfusion and metabolism. Quantitative analysis of bone turnover is of interest for evaluation of conditions with diffuse alteration to bone remodeling (such as primary hyperparathyroidism, renal osteodystrophy, and osteoporosis), and for assessment of bone perfusion, to assess bone (graft) vitality and osteonecrosis.
Quantitative bone scintigraphy was developed with 99mTc-MDP to study metabolic bone disorders with global effects on the skeleton. A simple parameter is 24-h whole-body bone retention, Kbone/(Kbone + Krenal) (8), where Kbone and Krenal denote the respective clearance of radioactivity from bone and renal plasma. These parameters can be measured by either a γ-camera or 24-h urine collection.
Although regional uptake measures are possible when combined with γ-camera imaging, whole-body bone retention represents an estimate for the entire skeleton. The whole-body bone retention method has several limitations. It assumes that radioactivity bound to bone will not dissociate from bone and that radioactivity in soft tissues is negligible at 24 h; these assumptions may not hold true. Nevertheless, in metabolic bone diseases, whole-body bone retention varies from normal values—decreasing in osteoporosis and increasing in renal osteodystrophy, Paget disease, osteomalacia, and primary hyperparathyroidism (3). When serial γ-camera imaging is combined with blood sampling, it is possible to measure 99mTc-MDP plasma clearance for the whole skeleton and for selected regions of interest (29). However, because of the variable degree of protein binding of 99mTc-MDP, ultrafiltration of plasma samples is necessary for accurate quantification. Despite general availability and relative model simplicity, quantitative bone scintigraphy has not gained widespread acceptance for clinical use, probably because of the availability of serum markers providing similar clinical information.
PET imaging with 18F-NaF has several advantages over quantitative methods using 99mTc-MDP bone scanning. In contrast to γ-camera imaging, including SPECT, PET allows for absolute quantification of radioactivity per measured volume. Quantitative analysis of 18F-NaF PET requires dynamic imaging and serial measurements of radioactivity in blood to obtain an input function, ideally from arterial blood. Hawkins et al. developed a 2-tissue, 3-compartment model (30), which in addition to a vascular compartment consists of bound and unbound bone compartments. The negatively charged fluoride anion, F−, diffuses into the extravascular (unbound bone) space. A certain fraction will then be further bound to the bone matrix and incorporated as fluoroapatite (F-apatite). The model has 5 variable parameters: 4 rate constants and 1 constant for the vascular compartment. The rate constants describing these processes are as follows: K1 and k2 for the forward and reverse capillary transport, k3 for the binding to the bone matrix, and k4 for the release reaction (Fig. 2). The magnitude of k4 is typically small in comparison to k2 and k3, indicating little dissociation of fluoride from the bone matrix. Within this model, the net transport of 18F-NaF into bone (commonly called Ki) is proportional to the term K1⋅k3/(k2 + k3), which can be obtained graphically (known as the Patlak plot). Accordingly, the fraction of tracer in the extravascular space that undergoes binding to bone mineral can be described by the term k3/(k2 + k3) (31).
An 18F-NaF 2-tissue-compartment model (3 compartments and 4 parameters).
An important advantage of compartmental modeling is that all individual rate constants (microparameters) and the net transport of 18F-NaF into bone (macroparameter) can be estimated; thus, bone blood flow, fluoride binding to bone mineral, and overall bone metabolism can be assessed at the same time. However, compartmental modeling approaches have demanding requirements. Dynamic PET requires a relatively long acquisition time of typically 60 min. Accordingly, the available field of view will be rather limited. Furthermore, arterial blood sampling and challenging data quality control are required to obtain precise estimates of microparameters. These issues have clearly limited clinical applications.
The computationally less demanding graphical method (Patlak plot) assumes that the release of radioactivity from the bone compartment is negligible. In many physiologic situations and low-turnover bone diseases, this assumption is valid. Since measurement errors are often lower with the graphical method than with compartment modeling, fewer subjects may be required to determine a statistically significant change (32). However, the Patlak plot provides only measurements for Ki and still requires a measured blood input function.
Various simplifications to this kinetic modeling approach have been proposed and tested. Arterial blood sampling can be avoided by substituting the arterial measurements with either population-derived input functions (33) or semi–population-derived input functions (34) requiring only a small number of additional venous blood samples. Image-derived input functions are an attractive alternative but are limited by the available field of view. Puri et al. examined various methods for quantification of Ki at the hip and lumbar spine, including 2-tissue-compartment 4-parameter, 2-tissue-compartment 3-parameter, and Patlak plots, and compared these with standardized uptake value (SUV) measurements (32). The measurement of SUV is comparatively simple as it can be obtained from static images without any blood sampling; however, it does not account for variable radiotracer clearance from the vascular space. Although statistically significant correlations between all quantification methods were reported, the data should be interpreted with caution as they were obtained only in healthy volunteers. Brenner et al. investigated compartmental modeling, Patlak plots, and SUV in a wide range of normal and pathologic bone conditions and confirmed strong correlations among methods (35). However, high variability was found for SUV measurements, indicating limited accuracy in cases of low metabolic bone turnover.
Dosimetry of 99mTc-MDP and 18F-NaF
In adults, the whole-body exposure from 18F-NaF administration is 0.024 mSv/MBq. Therefore, for a standard 370-MBq (10-mCi) 18F-NaF PET scan, the effective dose is 8.9 mSv. This compares with an adult 99mTc-MDP whole-body exposure of 0.0057 mSv/MBq, resulting in an effective dose of 5.3 mSv for a 925-MBq (25-mCi) administration (6). For 18F-NaF, the organ receiving the highest radiation dose is the bladder, whereas 99mTc-MDP has higher exposure to bone surfaces.
CLINICAL APPLICATIONS
Dynamic (3-phase) bone scanning involves an angiographic flow phase (typically using 1- to 3-s frames for 60 s after radiotracer injection), a soft-tissue phase (at 5–15 min), and a delayed phase (usually performed at 3–4 h) to allow for sufficient radiotracer clearance from soft tissues and radiotracer binding to bone (36). The second phase, often inaccurately described as the blood-pool phase, is obtained during a major shift of radiotracer from the blood to the extracellular space and then to target and nontarget tissues. Therefore, depending on the particular timing of this acquisition, commonly a significant amount of the radiotracer has already cleared from the blood, and the phase is therefore better referred to as the soft-tissue phase. Second- and third (delayed)-phase imaging can be performed using a whole-body technique to obtain whole-body soft-tissue and delayed bone-uptake images. Improvements to the technique have been made with SPECT imaging and the more recent combination of functional and anatomic imaging with SPECT/CT (37).
Typical quantitative dynamic PET imaging requires imaging of the same bed position for at least 60 min. For routine clinical applications, 18F-NaF PET (or PET/CT) could, however, be performed similarly to a 3-phase bone scan by obtaining a short (0–10 min) dynamic acquisition of an area of interest, which would represent both the angiographic flow and the soft-tissue phases of the 3-phase bone scan. Because 18F-NaF displays more rapid kinetics than does 99mTc-MDP, this dynamic scan can be relatively short. At around 60 min after injection, a whole-body acquisition representing the late-phase scan would follow. Although the utility of such an approach has not been tested and would depend on the particular technical characteristics of the PET scanner used, the approach would be expected to provide meaningful semiquantitative data that could replace a 3-phase bone scan at a fraction of time.
Bone Viability
Three-phase bone scanning is a suitable method to evaluate bone viability, an important factor for uncomplicated healing of vascularized bone grafts (38–40). Bone scintigraphy performed early (days 2–11) after surgery allows assessment of graft microperfusion, with graft survival reliant on intact blood flow during this critical period (38). Early bone scanning avoids false-positive results due to periosteal uptake along the graft surface and rare cases of late osteonecrosis presenting with uptake. Increased uptake in the graft predicts an uncomplicated healing course. Conversely, photopenia indicates vascular occlusion, which is followed by necrosis and graft failure (39). In a study investigating vascularized fibula grafts for repair of mandibular defects, uptake was present in 12 of 13 grafts with uncomplicated healing, whereas 3 failed grafts had decreased uptake (38). The technique can be improved with SPECT/CT imaging (Fig. 3) and semiquantitative analysis such as the measurement of transplant-to-cranium ratios, with values greater than 1.0 reported to have a high predictive value for successful healing (41). Three-phase bone scans were used to assess the vitality of vascularized femur allografts, with hyperemia indicating excellent perfusion and patency of transplanted vessels, and suitable uptake at 12 mo predicting a healthy graft (40). 99mTc-MDP bone scans have also been used to monitor new bone formation of the mandible during distraction osteogenesis for the treatment of hemimandibular abnormalities (42). A low level of bone uptake was suggestive of impending nonunion, requiring a decrease in the rate of distraction to allow healing.
99mTc-MDP bone scan for viability assessment of vascularized fibula graft in patient with osteonecrosis of jaw. On anterior, left, and right planar projections, angiographic (A) and soft-tissue (B) phases show nonspecific diffuse hyperemia overlying mandible due to surgical trauma. Delayed planar images (C) display focal increased uptake at genuine mandible near union sites of fibula graft. Transaxial and coronal SPECT/CT reveals photopenia in graft region (D). Regions of interest are defined in fibula graft (red) and calvarium (blue) on 3 consecutive slices to calculate graft-to-calvarium uptake ratio (41). Visual inspection and ratio result (0.72) are suggestive of nonviable graft.
In contrast to 3-phase bone scans, 18F-NaF PET allows quantitative measurements of bone blood flow and metabolism (43). Figure 4 demonstrates a case of a vascularized fibula graft after resection of a primary osteosarcoma of the femur shaft. Dynamic 18F-NaF PET of the transplant with proximal hypertrophic nonunion reveals significant differences in estimates for K1 and Ki, indicating impaired perfusion and metabolic function of the fibula transplant. Schliephake et al. used 18F-NaF PET to assess the viability of vascularized fibula grafts for mandibular reconstructions and reported that increased uptake (average of 19 d after surgery) within the graft and union sites, as compared with a cervical bone reference, indicted successful healing (44). In another study, graft failures were associated with near-zero fluoride influx into the fibula graft on 18F-NaF PET (45).
Plate fixation of vascularized fibula transplant (red arrow) after resection of osteosarcoma of right femur (A). Revision plate fixation was performed after fracture due to nonunion (yellow arrow). After injection of 370 MBq of 18F-NaF, dynamic PET study of mid thigh was performed for 60 min, followed by non–attenuation-corrected whole-body scan (B). Attenuation-corrected and axial slices (50–60 min) of hypertrophic nonunion (C) and fibular graft (D) areas show markedly increased uptake at nonunion and decreased uptake at level of fibula graft. Tissue time–activity curves (black squares) demonstrate rapid and sustained net-accumulation of radiotracer in nonunion but poor accumulation in fibula graft. Compartmental modeling was performed, providing estimates for total tissue activity (blue dots) and fraction of bound (red dots) and unbound (gray dots) radiotracer in tissue. At later time points, relationship of bound to unbound radiotracer is much higher in hypertrophic nonunion (C) than in failing graft (D).
Incorporation of cryopreserved allogenic bone graft material is uncertain as it has been shown to suffer from ineffective osteoinduction and osteoconduction (46). Previously, we used dynamic 18F-NaF PET to evaluate the biologic fate of allogenic bone grafts used to augment hip revision arthroplasties (47). That study revealed the presence of host bone formation in allogenic bone grafts early after surgery. Temmerman et al., using both 15O-H2O PET and 18F-NaF PET, investigated bone blood flow and metabolism in patients undergoing total hip revision arthroplasty using allogenic bone grafts (48). This pilot study revealed that areas of allogenic bone grafts displayed increased blood flow and metabolism as early as 2 wk after surgery that stabilized 3 mo after treatment, indicating new bone formation. Their observation of continued coupling of bone blood flow and metabolism was similar to our findings in allogenic bone grafts (47) and high-turnover bone disease (18). In an elegant PET study, Sörensen et al. evaluated bone blood flow (with 15O-H2O), bone blood volume (with 15O-carbon monoxide), and bone metabolism using 18F-NaF serially—8 d and 12 mo after surgery—to investigate incorporation of cryopreserved morselized allografts in total-hip arthroplasties (49). Their results indicated that blood flow and bone metabolism in allografts showed early increases that normalized 12 mo after surgery in patients with a successful clinical outcome. Although 18F-NaF PET is highly predictive of bone viability and surgical outcome, it remains uncertain whether observed increases in bone blood flow and metabolism can be attributed entirely to new bone formation and whether this bone formation is related to the host’s own osteoblasts or to osteoblasts originating from grafted tissues (50).
Osteonecrosis and Avascular Necrosis
Numerous studies have documented the usefulness of 3-phase bone scans for evaluation of avascular necrosis of the femoral head due to trauma, a slipped capital femoral epiphysis, steroid use, radiation effects, sickle cell disease, or Perthes disease, with accuracies exceeding 95% (Fig. 5) (9,36). Initial photopenia of the femoral head indicating interruption of blood flow is followed either by necrosis or subsequent reossification and healing response if revascularization occurs. Dasa et al. investigated 11 patients with nontraumatic osteonecrosis of the femoral head (51). Nine of 17 hips had increased acetabular 18F-NaF uptake not identified with bone scans, including SPECT, or with MR imaging, indicating that 18F-NaF may reveal changes earlier than traditional imaging. In a pilot study, Schiepers et al. used 18F-NaF PET/CT to predict healing of femoral head osteonecrosis by conservative measures based on blood flow estimates derived from 18F-NaF (52). A study performing 18F-NaF PET/CT after resurfacing arthoplasty found that lack of uptake in the femoral head was indicative of poor vascularity and subsequent osteonecrosis (53).
An 8-y-old boy with right hip pain and avascular necrosis of right femur head (Legg-Calve-Perthes disease). Anterior and posterior projection soft-tissue (A) and delayed-phase images (B) show focally decreased 99mTc-MDP uptake in right femoral head.
Serial Bone Imaging at Multiple Time Points
Pathophysiology of Bone Metastases
Disseminated tumor cells that successfully invade bone depend on adhesion mechanisms, interacting with the extracellular matrix, stromal cells, osteoblasts, osteoclasts, and endothelial cells to promote tumor cell survival (54). To form clinically overt metastases, tumor cells must be able to either stimulate cellular proliferation or attenuate micrometastatic mass dormancy programs. Phenotypically, bone metastases present as osteolytic (bone resorbing), osteoblastic (bone forming), or a mixture of both. As recently reviewed (55), these phenotypes are determined via a complex web of interactions among cancer cells, bone, and T-cells, a process that is mediated by many soluble factors and transcriptional activities that ultimately regulate osteolysis and osteoblastogenesis.
Staging of Bone Metastases
Bone scans are sensitive, cost-effective, whole-body imaging modalities for staging cancers that have a predilection for bone metastases (9), with overall sensitivity between 62% and 100% and specificity of 78%–100% (56). However, false-negative studies are commonly seen with osteolytic lesions, resulting in poor sensitivity (as low as 50% for myeloma). Use of SPECT/CT has been shown to improve specificity, with a reduced number of indeterminate bone lesions (compared with planar imaging) for staging of lung cancer bone metastases (37).
18F-FDG PET/CT for oncologic staging is highly sensitive for detection of bone metastases. For staging of lung cancer skeletal metastases, a metaanalysis that included 7 studies (1,794 patients) reported 18F-FDG PET sensitivity of 98% and specificity of 95%—superior to the conventional bone scan sensitivity of 87% and specificity of 82% (56). On imaging, osteoblastic, osteoclastic, or mixed phenotypes lead to mainly sclerotic, lytic, or mixed lesions, respectively. The predominant phenotype affects the detection potential of radiotracers. Thus, 18F-FDG PET has higher sensitivity for early marrow metastases and lytic lesions than do bone-seeking tracers.
18F-NaF PET is a highly sensitive method for detection of primary bone tumors (5) and osseous metastatic disease from a range of primary tumors, including lung, breast, prostate, thyroid, and squamous cell cancers of the head and neck (3,5,6). Generally, increased 18F-NaF uptake is found in sclerotic and mixed lesions and at cortical locations (57). 18F-NaF PET has superior sensitivity to 99mTc-labeled diphosphonate bone scanning using planar and SPECT protocols (5). When combined with hybrid PET/CT imaging, 18F-NaF was able to clarify many findings otherwise equivocal on conventional bone scanning (58). Krüger et al. compared 18F-NaF PET with 18F-FDG PET and 99mTc-MDP bone scanning in 126 patients with non–small cell lung cancer. 18F-NaF PET was superior in sensitivity to 99mTc-MDP and comparable to 18F-FDG PET (59). For biochemical relapse of prostate cancer, 18F-NaF appears to have greater diagnostic yield than 18F-FDG (60). In a comparative study between 18F-NaF PET and 18F-choline PET, 18F-NaF resulted in higher numbers of detected bone metastases, although detection of additional sites of disease did not alter management (61). 18F-NaF uptake may display a posttreatment flare phenomenon, similar to that reported in 99mTc-MDP bone scans (5).
Therapeutic Response Evaluation
In recent years, several promising systemic treatments for castration-resistant prostate cancer have been developed (62). In particular for progression after docetaxel chemotherapy, various mechanisms have been exploited, including cabazitaxel (second-generation taxane), abiraterone (targeting androgen-receptor signaling), 223Ra-chloride (α-particle emitter with calcium-mimetic high bone affinity), cabozantinib (tyrosine kinase inhibitor with activity against Met and vascular endothelial growth factor receptor 2), sipuleucel-T (immunotherapy and autologous dendritic cell therapy), and enzalutamide, many of which have shown survival benefits in clinical trials (62). Because development of these agents is expensive, objective proof of efficacy will be necessary using both biochemical and imaging biomarkers.
Conventional bone scans have several limitations for therapy response assessment. Unlike 18F-FDG, which targets the glucose consumption within tumors, bone-seeking radiotracers reflect the metabolic response of bone tissue to the presence of metastases. Thus, at best they represent an indirect measurement of tumor response. Consequently, even after successful systemic treatment of bone metastases, bone scans are known to improve very slowly, if at all (63). Other notable problems are false-negative results, a flare response leading to worsening of the scan appearance despite a clinical response to therapy, poor interobserver agreement, subjective interpretation, and inability to grade changes in intensity and extent (63,64). Also, certain chemotherapeutic approaches may directly interfere with bone remodeling. For example, the anti-RANKL (receptor activator of nuclear factor κB ligand) monoclonal antibody denosumab inhibits osteoclast function (65), which interferes with the ability to assess response on bone scans. Figure 6 illustrates that after denosumab treatment there is near-complete normalization of bone scan findings, although this may simply reflect inhibition of osteoclast function rather than an objective clinical response.
Breast cancer bone metastases identified on anterior and posterior projection whole-body bone scan in ribs, spine, pelvis, and left femur shaft (A). Treatment with denosumab results in near-normalization of scan findings (B), although with continued rise of tumor marker CA15-3. Three months after treatment, bone scan again identifies apparent disease progression (C).
A 99mTc-MDP bone scan index has been proposed as an imaging biomarker to improve the reproducibility of treatment response assessment. This index aims to quantify tumor burden as a percentage of the total skeletal mass of a reference man (63). Bone scan index and bone scan index doubling time measurements have been shown to correlate with outcome in clinical trials for castration-resistant prostate cancer (63,64). Computer-aided quantitative intensity normalization and segmentation of bone uptake compared with healthy controls has also been proposed to assess therapy response after cabozantinib treatment (measuring lesion area and numbers as well as radiotracer uptake per lesion) (64).
Although the ability to perform quantitative analysis in response evaluations is a key advantage of 18F-NaF PET over conventional bone scanning, it has rarely been applied in clinical trials. Dynamic 18F-NaF PET and kinetic modeling was used in breast cancer bone metastases to measure K1 and Ki, a potential technique for assessment of treatment response (66). Clearly, the requirement for a limited field of view for dynamic 18F-NaF PET is undesirable. Therefore, semiquantitative analyses of SUVs obtained from static whole-body 18F-NaF PET acquisitions were used to assess response to 223Ra-dichloride treatment. In a small pilot study, 18F-NaF SUV appeared to have predictive value for evaluating the response of prostate cancer bone metastases, whereas qualitative (visual) interpretation tended to be less valuable (67). These initial results demonstrate the potential for quantitative approaches to monitor treatment response using 18F-NaF PET.
Benign Metabolic Bone Disorders
Paget disease is an idiopathic disorder characterized by accelerated bone turnover with abnormally increased osseous formation and resorption. Quantitative methods on bone scintigraphy provide noninvasive measurements of bone turnover that have been shown to correspond to serum and urinary markers of bone turnover (68,69). Polyostotic Paget disease is characterized by increased global skeletal uptake, compared with healthy controls (69). The whole-body bone index is a parameter developed for objective characterization of the extent of polyostotic Paget disease to allow comparison on serial studies to assess treatment response (68). In metabolic bone disorders such as primary hyperparathyroidism, bone scans typically show diffuse increased uptake throughout the skeleton and may result in a “superscan” appearance (9). Also, quantitative bone scintigraphy demonstrated increased bone turnover in 51% of patients with primary hyperparathyroidism and in 78% of patients with thyrotoxicosis, compared with healthy controls (70).
Paget disease has been studied with dynamic 18F-NaF PET/CT using compartmental modeling, which confirmed increased net fluoride influx to pagetic bone; involved vertebral bodies displayed increased K1 and Ki values compared with normal vertebrae (71). In hereditary hyperostosis cranialis interna, 18F-NaF PET/CT was used to quantify bone metabolism in the skull base of affected patients and provided information about disease severity and pattern of involvement (72). Dynamic 18F-NaF PET has also been used to study metabolic bone diseases. In renal osteodystrophy, a common cause for secondary hyperparathyroidism, there was increased bone metabolism (increased Ki) in affected patients compared with healthy subjects (23). After parathyroidectomy and medical management, bone metabolism (Ki) decreased between 30% and 40%, demonstrating the ability to monitor treatment response. In an experimental porcine model of parathyroid hormone–related high-turnover bone disease, dynamic 18F-NaF PET allowed the detection of high-turnover osteopenia after gastrectomy in mini pigs (73,74). Furthermore, the combination with quantitative CT bone densitometry allowed normalization of metabolic changes by the specific bone mass, thus increasing the discriminatory power in metabolic bone diseases (74).
Quantitative dynamic 18F-NaF PET/CT has also been performed for the investigation of low-turnover bone diseases, including osteoporosis (75), and to assess age-related changes in pre- and postmenopausal women (76). In a large study performed by Frost et al., dynamic 18F-NaF PET and bone mineral densitometry were performed on 72 postmenopausal women classified as having normal, osteopenic, or osteoporotic bones (31). Bone metabolism (Ki) and fluoride binding to bone mineral [k3/(k2 + k3)] were significantly reduced in osteoporosis, whereas at the same time biochemical markers of bone turnover (bone-specific alkaline phosphatase) were increased. This somewhat surprising result highlights the importance of regional measurements of bone turnover (from imaging) to improve the understanding of metabolic bone diseases.
Bisphosphonate Treatment-Response Evaluation
The availability of effective treatments for Paget disease, osteoporosis, and bone metastases has led to renewed interest in quantitative imaging of bone metabolism to assess response. Bisphosphonates are biologically stable analogs of pyrophosphates that have a high affinity for hydroxyapatite and induce apoptosis of osteoclasts, thereby inhibiting bone resorption (77). In monoostotic Paget disease, serum biomarkers are often within the reference range. Accordingly, regional bone scanning has been used to assess response to bisphosphonate treatments, including comparative analysis of bisphosphonate (risedronate and tiludronate) treatments (78).
18F-NaF PET offers the ability to monitor the response to bisphosphonate treatment quantitatively. In 14 patients with Paget disease, bone metabolism as measured by Ki (obtained from nonlinear regression and Patlak analysis) and by SUV obtained from static 18F-NaF PET significantly decreased after bisphosphonate treatment (79). This study indicated that simple SUV measurements may be sufficient for monitoring disease response in metabolic bone diseases. After alendronate treatment of osteoporosis, decreases in serum alkaline phosphatase were seen, along with decreasing 18F-NaF SUV measurements in lumbar spine and femoral sites, associated with increases in bone mineral density on dual-energy x-ray absorptiometry (80). Therefore, simplified measurement of SUV on static 18F-NaF PET/CT promises to improve clinical application.
CONCLUSION
Because of similarities between the uptake mechanisms of 99mTc-labeled diphosphonates and 18F-NaF, routine clinical use of whole-body 18F-NaF PET can be quickly implemented. Quantitative measurements of bone blood flow and metabolism with 18F-NaF PET have been instrumental to our understanding of metabolic bone diseases and bone regeneration after surgical interventions. Treatment-induced changes to bone metabolism provide an impetus for using 18F-NaF PET to assess response to antiresorptive bisphosphonate treatments and novel therapies of bone metastases. Clearly, the necessity for dynamic image acquisition has limited clinical applications of quantitative 18F-NaF PET, an otherwise highly sensitive and accurate imaging approach. Initial reports indicating that simplified 18F-NaF SUV uptake measurements may be suitable substitutes for more complex kinetic modeling are therefore encouraging.
Footnotes
Published online Mar. 12, 2013.
Learning Objectives: On successful completion of this activity, participants should be able to (1) appreciate the physiologic mechanisms underlying uptake of bone-seeking radiopharmaceuticals used to assess bone perfusion and turnover and note the differences between 18F-NaF and 99mTc-labeled diphosphonates; (2) discuss the use of bone scintigraphy for evaluation of bone viability and metabolic bone disorders and appreciate the potential role of quantitative dynamic 18F-NaF PET for evaluation of response to therapy; and (3) discuss indications for 99mTc-labeled diphosphonate bone scans and 18F-NaF PET/CT for oncologic staging and appreciate quantitative methods on static and dynamic bone imaging as imaging biomarkers of treatment response using novel systemic therapies for metastatic castrate-resistant prostate cancer as a model.
Financial Disclosure: The authors of this article have indicated no relevant relationships that could be perceived as a real or apparent conflict of interest.
CME Credit: SNMMI is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing education for physicians. SNMMI designates each JNM continuing education article for a maximum of 2.0 AMA PRA Category 1 Credits. Physicians should claim only credit commensurate with the extent of their participation in the activity. For CE credit, participants can access this activity through the SNMMI Web site (http://www.snmmi.org/ce_online) through April 2016.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- Received for publication January 23, 2013.
- Accepted for publication February 28, 2013.