Abstract
Prostate-specific membrane antigen (PSMA)–targeting α-radiation therapy (TAT) is an emerging treatment modality for metastatic castration-resistant prostate cancer. There is a subgroup of patients with poor response despite sufficient expression of PSMA in their tumors. The aim of this work was to characterize PSMA-TAT–nonresponding lesions by targeted next-generation sequencing. Methods: Of 60 patients treated with 225Ac-PSMA-617, we identified 10 patients who presented with a poor response despite sufficient tumor uptake in PSMA PET/CT. We were able to perform CT-guided biopsies with histologic validation of the nonresponding lesions in 7 of these nonresponding patients. Specimens were analyzed by targeted next-generation sequencing interrogating 37 DNA damage-repair–associated genes. Results: In the 7 tumor samples analyzed, we found a total of 15 whole-gene deletions, deleterious or presumably deleterious mutations affecting TP53 (n = 3), CHEK2 (n = 2), ATM (n = 2), and BRCA1, BRCA2, PALB2, MSH2, MSH6, NBN, FANCB, and PMS1 (n = 1 each). The average number of deleterious or presumably deleterious mutations was 2.2 (range, 0–6) per patient. In addition, several variants of unknown significance in ATM, BRCA1, MSH2, SLX4, ERCC, and various FANC genes were detected. Conclusion: Patients with resistance to PSMA-TAT despite PSMA positivity frequently harbor mutations in DNA damage-repair and checkpoint genes. Although the causal role of these alterations in the patient outcome remains to be determined, our findings encourage future studies combining PSMA-TAT and DNA damage-repair–targeting agents such as poly(ADP-ribose)-polymerase inhibitors.
Prostate-specific membrane antigen (PSMA)–targeting radioligand therapy is an emerging and promising approach to treating metastatic castration-resistant prostate cancer. A phase 2 trial found a serum prostate-specific antigen response rate of 57% for the β-particle–emitting radiopharmaceutical 177Lu-PSMA-617 (1). High antitumor activity was also reported for the α-particle–emitting 225Ac-PSMA-617; a recent study, however, relying on different inclusion criteria, achieved prostate-specific antigen responses in 63% (2). Other drugs that have been evaluated in phase 2 or 3 trials for therapy of prostate cancer during the last decade achieved prostate-specific antigen response rates of only 54% (enzalutamide), 39% (cabazitaxel), 29% (abiraterone), and 10% (223RaCl2) (3).
α-radiation is characterized by a high-linear-energy transfer causing 2,000–7,000 ion pairs per micrometer in water, that is, 1 ionization event every 2 nm. Because the diameter of the DNA double-helix is about 2 nm, the traversal of a single α-particle is enough to induce double-stranded, often blunt-ended, DNA breaks (4). β-radiation results in fewer than 20 ion pairs per micrometer, and the traversal of a single β-particle causes only single-stranded DNA breaks, whereas higher absorbed doses are needed to achieve double-strand breaks, and then often with cohesive ends (4). Taking into account that it is more challenging for a cell to repair a blunt-ended double-strand break than a sticky-ended or only single-stranded DNA break, it seems reasonable that fewer patients should be resistant against PSMA-targeted α-radiation therapy (TAT) (5). However, the reported subgroup of 37% of patients with a poor response or early resistance against 225Ac-PSMA-617 is still surprisingly large (2).
PSMA PET/CT is routinely used as a stratification tool to select patients with PSMA-positive tumor phenotypes toward PSMA-TAT. However, only a moderate correlation of pretherapy SUVs, tumor-absorbed dose, and treatment response was observed (6,7). Obviously, in addition to quantitative tumor targeting, accessory factors are influencing response to PSMA-TAT. Preclinical and early clinical studies reported that particular DNA damage-repair–associated gene mutations (DRMs) can either increase or decrease the radiosensitivity of prostate cancers (8–14) and thus might represent one of these cofactors.
In parallel to the development of PSMA-TAT, the poly(ADP-ribose)-polymerase (PARP) inhibitor olaparib has been evaluated for metastatic castration-resistant prostate cancer and received a Food and Drug Administration breakthrough designation for patients with a germline or somatic mutation of ATM or BRCA1/2. Antitumor activity was also observed in patients positive for other DRMs (15,16). Therefore, genetic characterization of our patients was warranted by clinical indication.
Given the important role of DNA damage-repair gene alterations in the response to DNA-damaging agents, including PSMA-TAT, and the clinical relevance for the use of PARP inhibitors, the aim of this pilot study was to evaluate the frequency of these defects in patients insufficiently responding to 225Ac-PSMA-617 therapy.
MATERIALS AND METHODS
Patient Characteristics
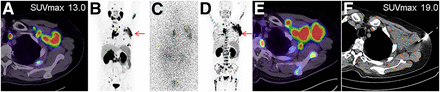
The cohort was selected from 60 patients treated with 225Ac-PSMA-617; 40 of them have been described previously (2). In a first step, patients with increasing prostate-specific antigen levels and progression of radiologically evaluable lesions on treatment were identified. Retrospective reevaluation of PSMA PET in comparison to a second imaging modality identified 10 patients for whom the poor response could be related to a faint uptake of PSMA ligands in at least some viable tumor lesions (Fig. 1), resulting in a collective with homogeneously PSMA-positive lesions. Of 10 patients with a poor response despite sufficient PSMA expression, that is, an SUV of more than 10 (equals ∼2-fold liver and 1.2-fold salivary gland uptake), 7 patients with suitable lesions for biopsy (lymph node or soft-tissue metastases or a large osteolytic bone lesion) agreed to tissue sampling and 3 patients (after being informed about the challenging location of their lesions) refused biopsy. A flowchart (Fig. 1) demonstrates patient selection. The clinical indication to perform these biopsies was to select patients suitable for treatment with either olaparib (if DRM-positive) or platin-containing chemotherapy (in cases of dedifferentiation or neuroendocrine transdifferentiation). Detailed patient characteristics are summarized in Table 1.
(Left) Flowchart of patient selection. Biopsies were taken only from tumor lesions with nonresponse despite high uptake in PSMA PET scans. (Right) Patient with several 18F-FDG–positive (viable) and visually concordant PSMA-positive lesions (green arrows) at baseline. After 3 cycles of PSMA-targeted therapy, residual lesions (18F-FDG–positive) were demasked to be PSMA-negative (red arrows). MIP = maximum-intensity projection.
Patient Characteristics
All patients provided written informed consent. The retrospective evaluation of data acquired with clinical indication during PSMA therapy (vote S-321-2012) and the genetic analysis (votes S-085/2012 and S-051/2017) were approved by the ethics committee of the University Heidelberg and performed in accordance with the updated Declaration of Helsinki.
PSMA PET/CT, Treatment Emission Scans, and Imaging-Guided Biopsies
All PSMA PET/CT was performed with a clinical routine protocol on a Biograph mCT Flow scanner (Siemens) using 68Ga-PSMA-11.
Posttherapy 225Ac-PSMA-617 scans were acquired using the 440-keV γ-coemission of 213Bi (26% emission probability), the 218-keV γ-coemission of 221Fr (12%), and the bremsstrahlung of 209Pb with a scan speed of 10 cm/min on a 2.54-cm-crystal (1-in) γ-camera (Hawkeye; GE Healthcare) equipped with a high-energy collimator.
CT-guided biopsy of one of the most PSMA-avid tumor sites (liver, 2 retroperitoneal lymph nodes, os ilium, skin, axillary lymph node) was performed using PSMA PET/CT for localization (Fig. 2). Each biopsy was performed according to interventional standard procedure, adequately adapted to patient needs. A mean of 5 (range, 2–10) core-cut specimens (17-gauge; length, 15–22 mm) were taken and fixed in buffered formalin. Conventional histopathologic examination validated that viable tumor tissue was successfully sampled in all cases.
(A) In PSMA PET/CT, residual lymph node metastasis with SUV of 37.7 remained after PSMA-targeted α-therapy. (B) In contrast-enhanced CT, location of metastasis was clearly delineated (encircled). (C and D) CT-guided biopsy was performed (C), and histopathologic validation of tumor lesion was obtained, for example, per PSMA immunohistochemistry (D).
Histopathology and PSMA Immunostaining
After hematoxylin and eosin staining, representative sections were stained for PSMA by immunohistochemistry. Sections were deparaffinized in xylene and rehydrated in a graded ethanol series. Antigen was retrieved with a steam cooker using retrieval buffer (Target Retrieval Solution; Dako). A mouse monoclonal antibody against PSMA (clone 3E6; Dako) was used at a 1:100 dilution and incubated overnight at 4°C. Immunodetection was performed using the Histostain-Plus detection kit (Invitrogen) according to the manufacturer’s recommendations. Stained sections were scanned using a Nanozoomer 2.0-HT Scansystem (Hamamatsu Photonics).
Targeted Next-Generation Sequencing
Two separate targeted next-generation-sequencing panels, the Oncomine BRCA Research Assay (Thermo Fisher Scientific) and a proprietary panel (HDRv1) that was developed at our institution, were used for mutation detection in the following 37 genes involved in DNA damage, checkpoint signaling, or DNA damage repair: BRCA1, BRCA2, ATM, ATR, BARD1, BRIP1, CHEK1, CHEK2, FAM175A, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, ERCC2, ERCC4, ERCC5, MLH1, MSH2, MSH6, PALB2, PMS1, PMS2, RAD50, RAD51C, RAD51D, RECQL4, MRE11A, NBN, SLX4, TP53, and XRCC2.
Library preparation and semiconductor sequencing were performed as described earlier (17,18). In short, for both panels, amplicon libraries were prepared using 2 primer pools with 5 or 10 ng of DNA and the Ion AmpliSeq Library Kit, version 2.0 (Thermo Fisher Scientific). Subsequent to polymerase chain reaction amplification, primer end sequences were partially digested using FuPa reagent (Life Technologies) and ligated to barcoded sequencing adapters (Ion Xpress Barcode Adapters; Life Technologies). The final libraries were purified using AMPure XP magnetic beads (Beckman Coulter) and quantified using the Ion Library Quantitation Kit (Thermo Fisher Scientific) on a StepOne system (Thermo Fisher Scientific). The individual libraries were diluted to a final concentration of 50 pM, combined, and processed to library amplification and enrichment on the Ion Chef system together with the Ion 520 and Ion 530 Kit-Chef (both Thermo Fisher Scientific). The processed libraries were then loaded on an Ion 530 chip, generating a mean coverage of 1,000- to 3,000-fold per amplicon.
Data analysis was performed using the Ion Torrent Suite Software (version 5.8.0) as described previously (19). After base calling, the reads were aligned against the human genome (hg19) using the TMAP algorithm within the Torrent Suite. Variant calling was performed with the variant caller plugin (version 5.8.7-1), applying a frequency of more than 5% and a minimum coverage of 200 reads. Variant annotation was performed using Annovar (20). Annotations included information about nucleotide and amino acid changes of RefSeq annotated genes, COSMIC, and dbSNP entries, as well as detection of possible splice site mutations. For data interpretation and verification, the aligned reads were visualized using the IGV browser (Broad Institute) (21).
RESULTS
Potentially DNA-Damaging Pretreatments
The patient collective analyzed here presents a selection (n = 7 of initially 60) of advanced-stage patients. All patients were previously treated with standard androgen deprivation therapy (5 patients, GnRH axis and nonsteroidal antiandrogen; 1 patient, antiandrogen; and 1 patient, GnRH axis only). Seven of 7 patients received abiraterone, and 5 of 7 patients also received enzalutamide. All patients had previously received docetaxel, and 4 of them additionally received cabazitaxel. Olaparib was never considered before PSMA-TAT. None of these standard treatment modalities directly interfere with DNA integrity or repair.
In addition, all patients had a history of external-beam radiotherapy: 4 of 7 on the prostatic bed, 5 of 7 on the pelvic lymphatic drainage, and 5 of 7 focusing on symptomatic bone lesions. Previous systemic β-emitter–based radioligand therapy was administered to 4 of 7 patients, 2 of them with nonresponse directly from the start and 2 with acquired resistance after 3 and 7 mo of 177Lu-PSMA617. These previous therapies are inherently promutagenic and could affect tumor mutational burden; they may also induce some selection bias toward radioresistant tumors even in advance of PSMA-TAT. Such heavily pretreated patients inherently bear a high risk for interlesion heterogeneity, which was addressed by selective, imaging-guided biopsies dedicated to PSMA-positive but PSMA-TAT–resistant lesions (Fig. 3).
Intrapatient tumor heterogeneity makes tissue sampling of the most appropriate index lesion challenging. (A) Baseline PSMA PET/CT demonstrated group of 3 lymph nodes with homogeneously intense uptake with SUVs of 29.7–35.5 (arrows). (B) After 225Ac-PSMA therapy, 2 lesions with SUVs of 29.7 and 32.0 showed morphologic response (green arrows), but index lesion with highest initial uptake (SUV, 35.5) had increased size (red arrow) and persisting PSMA uptake (SUV, 30.0). (C) This lesion (encircled) was chosen for imaging-guided biopsy.
Tumor-Phenotype Evaluation per PSMA PET/CT Before and After Therapy
The mean SUVmax of 5 progressive index lesions was 21.0 ± 10.1 (median, 17.8) before therapy and 23.3 ± 8.5 (median, 24.5) after 225Ac-PSMA617 therapy. Thus, the tumor uptake of PSMA ligands rather increased under PSMA-targeting therapy, indicating that nonresponse to the radiopharmaceutical was caused by radioresistance and not by insufficient expression of the target receptor. In 2 patients, new lesions occurred under PSMA-TAT; these newly occurring lesions were still sufficiently PSMA-positive (SUVmax, 17 and 37). In patients who presented with a mixed response, that is, simultaneous remission of some lesions and progression of others, there was no relevant difference in the mean SUVs of responding versus nonresponding lesions; there was even a patient in whom the nonresponding lesions showed a trend toward higher SUVs than the responding ones (Fig. 4).
(A and B) Baseline PSMA PET demonstrated intense uptake of PSMA ligand in axillary lymph node bulk. (C) Planar emission scan of 225Ac-PSMA validated positive tumor targeting during therapy. (D and E) Restaging PSMA PET revealed morphologic progression and even increased PSMA expression of lesion. (F) Consequently, lesion was chosen for CT-guided biopsy.
Next-Generation Sequencing
The 7 patients not responding to PSMA-TAT were further analyzed for possible genetic alterations in genes related to the DNA damage-repair system (Table 2). The most abundant alterations causing a loss of function were found in TP53 (3/7 patients), ATM (2/7), and CHEK2 (2/7), with 1 patient having a TP53 and CHEK2 commutation. Although both deleterious mutations and whole-gene deletions were seen for TP53 and ATM, both patients with a CHEK2 alteration harbored a deleterious mutation. Further loss-of-function mutations or whole-gene deletion was detected once in the analyzed sample set for BRCA1, PMS1, and PALB2 and in the analyzed sample set for FANCB, NBN, MSH2, MSH6, and BRCA2, respectively.
Summary of Observed Gene Defects and Previous Exposure to 225Ac-PSMA617
In total, we found at least 1 genetic alteration negatively affecting the DNA damage-repair system in 6 of 7 patients. In 1 tumor (patient 3) low-level amplifications of ATR, BRIP1, and SLX4 were detected additionally.
Treatment Activity Versus Number of Mutations
Correlating the genetic alterations with the parameters of the PSMA-TAT therapy, we observed that the 2 patients who had received the highest 225Ac treatment dose were harboring the lowest number of gene deletions (20 MBq/deletion of PALB2 and 22 MBq/no gene deletion and only a variant of unknown significance within MSH2). In contrast, the patient with the lowest exposure to α-radiation presented with 2 deletions (12 MBq/ATM and TP53 deletion). Multiple gene deletions were found in patients who had received an intermediate activity of 225Ac (14–18 MBq/3–4 genes deleted; Table 2). Thus, we found no indication that the number of (DNA damage-repair) gene deletions after PSMA-TAT correlated with the dose of α-therapy itself.
DISCUSSION
It is well accepted that PSMA-targeting therapies, regardless of whether they are based on 177Lu or 225Ac and regardless of whether they are based on small molecules or antibody–drug conjugates, are not efficient in tumors with poor expression of the target receptor PSMA. However, there are also nonresponding lesions despite sufficient PSMA expression. The underlying resistance mechanisms of these tumors have not yet been identified.
In this work, tumor lesions progressing under 225Ac-PSMA-617 despite intense expression of the target receptor PSMA were genetically characterized by targeted next-generation sequencing of 37 DNA damage-repair–associated genes to evaluate whether these patients may be candidates for treatment with PARP inhibitors. We found at least one, and often even multiple (average of 2.2 per patient), functional relevant deleterious mutations in 6 of 7 patients analyzed (86%).
There was no clinical indication to evaluate patients still sufficiently responding to PSMA-TAT. Without a control group evaluating the mutational burden of responding lesions in advance of therapy (the primary limitation of our study design), the interpretation of our results depends on comparison with literature data. In general, the pretest probability of finding such mutations increases with more advanced tumor stages, with the number of previous therapies, and with the number of analyzed target genes (22). The characteristics of our cohort are similar to those of the patients evaluated with a 113-gene targeted next-generation sequencing panel in the TOPARP trial (16) and with a 25-gene panel focused on DNA damage repair in a 223Ra study (23). These studies reported functional relevant mutations in 16 of 49 (33%) and 10 of 28 (38%) patients, respectively. In comparison to these previous reports, the frequency of 86% DRMs appears to be enriched in our patients. One simple explanation could be that the treatment with a promutagenic radiopharmaceutical is responsible for this finding. However, this idea is not supported by our observation that the number of genetic alterations did not correlate with the cumulative dose of 225Ac-PSMA-617. Nevertheless, previous β-radiation with 90Y/177Lu-PSMA-617 in 4 of our patients presents a mentionable difference from historical controls, and the promutagenic potential of this treatment line still remains to be determined.
Eventually, the focus on highly PSMA target–positive cancers, stratified by PSMA PET/CT already in advance of PSMA-TAT, could introduce some selection bias. Several groups found high PSMA expression measured by either immunohistochemistry (24–26) or PSMA PET (27) to correlate with traditional negative prognostic factors such as Gleason score, T-stage, and the d’Amico criteria and to serve as an independent predictor of prostate cancer recurrence and progression. A similar correlation between Gleason score, tumor stage, and prognosis was found for patients with DRMs (28,29). One recent work (relying on immunohistochemistry) found higher PSMA expression in BRCA2- and ATM-deficient than in unselected tumor samples (30). Thus, a somehow increased overlap between PSMA PET–positive patients and DRM carriers would be reasonable.
Despite all these potential selection biases, the high frequency of DRMs in radioresistant patients is still a surprise; one might rather expect that patients with a truncated DNA damage-repair pathway should be exceptionally sensitive against radiopharmaceuticals. However, because of the complexity of DNA damage repair, requiring activation of a cascade of several related factors, basic research is more controversial. ATM and CHEK2 serve as sensors of DNA integrity and are known activators of TP53, which serves as a signaling checkpoint to discontinue mitosis until DNA integrity can be restored. Loss of such DNA damage recognition-and-signaling genes has often been described to promote radioresistance (10–12,31,32). In contrast, the protein product of the BRCA2 gene plays a key role in the effector downstream of DNA damage repair and deficiency at this point of the cascade, commonly translating into increased radiosensitivity (12,13,33,34). In the 223Ra study, 3 of 10 mutation carriers were harboring a BRCA2 mutation (also the only mutation reported more than once) and belonged to the best responders toward the following 223Ra therapy (23). Also in other epidemiologic studies, BRCA2 was found among the most common mutations (16,22). In contrast, we found 7 deleterious mutations of ATM, CHEK2, or TP53 but only a single BRCA2 loss in our PSMA-TAT–resistant patients; thus, deficient damage recognition and signaling was overrepresented in comparison to damage-repair–related mutations. This pilot study was not designed to explore causal relationships; however, on the basis of our interesting observation, we encourage consideration of DRMs as a control variable in future PSMA trials to evaluate their respective value as prognostic biomarkers.
Release of endogenous cancer-specific antigens from radiation-induced necrosis can lead to a generalized tumor immune response to these antigens via cross-talk mechanisms, the so-called abscopal effect. Thus, combined radioimmunotherapy was already considered generally beneficial (35). Recent data suggested that a high tumor mutational burden in prostate cancer might further improve its response to immunotherapies (36,37). Encouraging clinical results have also been demonstrated for combination of 177Lu-PSMA-617 with radiosensitizers (38). In addition to their specific antitumor activity in ATM- and BRCA1/2-mutated patients, PARP inhibitors also act as unspecific radiosensitizers (13,14,39). Thus, regardless of whether the observed coincidence of DRMs and resistance to PSMA-TAT despite PSMA positivity reflects tumor biology or other selection biases, it seems reasonable to speculate that the combination of PSMA-TAT with immunotherapy or PARP inhibitors may act complementarily.
CONCLUSION
We found an unexpected high frequency of DRMs in patients insufficiently responding to PSMA-TAT monotherapy. This observation provides a good rationale to evaluate combination therapies of PSMA-TAT with PARP inhibitors or immunotherapies. Additional studies are required to explore the value of particular DRMs that may have potential as prognostic biomarkers in advance of PSMA-TAT.
DISCLOSURE
Uwe Haberkorn and Clemens Kratochwil have a patent application for PSMA-617. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is resistance to PSMA-targeted α-therapy associated with mutations in genes related to DNA damage repair?
PERTINENT FINDINGS: In comparison to the literature, the frequency of DNA damage-recognition and signaling-checkpoint genes appears increased in patients with nonresponse to radioligand therapy despite high uptake in PSMA PET.
IMPLICATIONS FOR PATIENT CARE: Combination therapies of PSMA-TAT and PARP inhibitors or immunotherapies might act complementarily. Particular mutations could have potential as prognostic biomarkers in advance of PSMA therapy.
Footnotes
Published online Oct. 10, 2019.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 31, 2019.
- Accepted for publication September 20, 2019.