Abstract
To protect bone marrow from overirradiation, the maximum permissible activity (MPA) of 131I to treat thyroid cancer is that which limits the absorbed dose to blood (as a surrogate of marrow) to less than 200 cGy. The conventional approach (method 1) requires repeated γ-camera whole-body measurements along with blood samples. We sought to determine whether reliable MPA values can be obtained by simplified procedures. Methods: Data acquired over multiple time points were examined retrospectively for 65 thyroid cancer patients, referred to determine 131I uptake and MPA for initial treatment after thyroidectomy (n = 39), including 17 patients with compromised renal function and 22 patients with known (n = 16) or suspected (n = 6) metastases. The total absorbed dose to blood (DTotal) was the sum of mean whole-body γ-ray dose component (Dγ) from uncollimated γ-camera measurements and dose due to β emissions (Dβ) from blood samples. Method 2 estimated DTotal from Dβ alone, method 3 estimated DTotal from Dγ alone, and method 4 estimated DTotal from a single 48-h γ-camera measurement. MPA was computed as 200 cGy/DTotal for each DTotal estimate. Results: Method 2 had the strongest correlation with conventional method 1 (r = 0.98) and values similar to method 1 (21.0 ± 13.7 cGy/GBq vs. 21.0 ± 14.1 cGy/GBq, P = 0.11), whereas method 3 had a weaker (P = 0.001) correlation (r = 0.94) and method 4 had the weakest (P < 0.0001) correlation (r = 0.69) and lower dose (16.3 ± 14.8 cGy/GBq, P < 0.0001). Consequently, correlation with method 1 MPA was strongest for method 2 MPA (r = 0.99) and weakest for method 4 (r = 0. 75). Method 2 and method 1 values agreed equally well regardless of whether patients had been treated with 131I previously or had abnormal renal function. Conclusion: Because MPA based on blood measurements alone is comparable to MPA obtained with combined body counting and blood sampling, blood measurements alone are sufficient for determining MPA.
Thyroid cancer is the most rapidly increasing malignancy diagnosis in the United Sates (1,2). The most common form of thyroid cancer is differentiated thyroid cancer, the recurrence rate of which is estimated to be 30% (3). Typical treatment for thyroid cancer is total thyroidectomy followed by administration of 131I to ablate thyroid remnants to reduce the risk of disease recurrence (4).
Some institutions administer empiric 131I activity to all patients, but doing so delivers excessive radiation dose to some patients (5). The conventional approach to estimating 131I maximum permissible activity (MPA) that can be administered to limit blood dose to less than 200 cGy (200 rad) involves repeated γ-camera measurements and blood sampling over 5 d (6,7), and longer for patients with decreased renal clearance (8). Additional restrictions include limiting whole-body retention at 48 h to less than 4.4 GBq and to less than 3 GBq for patients with diffuse pulmonary disease (9,10).
The conventional approach to estimating MPA assumes total dose to blood (DTotal) is the sum of dose to blood from 131I β-emissions (Dβ), measured from blood samples, and γ-ray dose to blood from all other organs due to 131I γ-emissions (Dγ), estimated from γ-camera measurements. Some institutions find this conventional MPA approach inconvenient. Alternative techniques reduce or eliminate blood sampling altogether (11,12), estimating MPA from a single body counting radiation measurement (13).
Our investigation was undertaken to determine whether alternate MPA methods agree with the conventional method. Because some institutions routinely administer 7.4 GBq (200 mCi) of 131I for metastatic disease (13,14), despite findings that 7.4 GBq of 131I would exceed 200 cGy of blood dose in 19% of patients (5), we also evaluated which methods indicate the need to administer less than 7.4 GBq. In addition, we investigated effects of previous radioiodine treatment or abnormal renal function on different MPA methods.
MATERIALS AND METHODS
Patients
Data were examined retrospectively for 65 patients (age, 60 ± 14 y; 31 women, 34 men), studied from January 2004 through October 2016. Thirty-nine patients were referred to determine 131I uptake and MPA for initial treatment after thyroidectomy, including 17 patients with compromised renal function and 22 patients with known (n = 16) or suspected (n = 6) metastases. Two patients were studied twice, and 2 patients were studied 3 times, so that there were a total of 71 studies. Standard patient preparation procedures before beginning 131I dosimetry consisted of counseling patients to maintain a strict low-iodine diet for 2 wk before beginning the procedure and ensuring that they were either hypothyroid with thyroid-stimulating hormone levels greater than 0.25 μIU/mL (n = 27 studies) (15), or had received thyrotropin alfa (0.9 mg) on 2 consecutive days before 131I administration (n = 44 studies). Determination of whether patients had abnormal renal function was assessed by examining laboratory reports, defined as blood urea nitrogen or creatinine levels exceeding referring laboratories’ internally established normal limits.
The Institutional Review Board approved this retrospective study, and the requirement to obtain informed consent was waived. All data were handled in compliance with the Health Insurance Portability and Accountability Act of 1996.
Data Acquisition
The dose to blood was determined by acquiring data using conventional techniques (6,7). On the first day, 3–4 mL of blood were withdrawn before 131I administration and counted in a well-counter (2470 γ-counter [2″ crystal]; Perkin Elmer) for initial background activity determination. An uncollimated γ-camera detector was peaked on 131I (15% energy window) and positioned at a fixed location. All subsequent camera measurements were performed on the same γ-camera with fixed camera configuration and energy settings. A 131I calibration source verified consistent camera operation from 1 whole-body counting session to another.
One hour after 131I administration, the patient began whole-body counting. Background and calibration counts were obtained at the beginning of each camera counting session, followed by anterior and posterior counts of the patient. Initial patient counts were acquired 1 h after 131I administration, measurement of which constituted 100% of counts. This process was repeated at 4, 24, and 48 h. At 48 h, background-corrected and decay-corrected conjugate-view counts were used to estimate the percentage retained activity. If less than 60% of initial activity was retained, patients returned at 72 and 96 h; if 60% or more was retained, patients returned at 96 and 144 h, but not at 72 h. All counts were acquired for 1 min.
Blood samples (3–4 mL) were drawn within 1 h of each camera counting session. At the end of all counting sessions (i.e., 96–144 h after capsule ingestion), 1-mL aliquots of whole blood collected throughout the week were counted in a well-counter, along with a 131I calibration capsule and a 1-mL water sample for background.
Data Processing
All measurements were entered into a spreadsheet (Excel; Microsoft Inc.). Counts were corrected for background radiation and radioactive decay to the time of administration.
Method 1: Conventional Approach
To accommodate multiple biologic compartments with different excretion rates (7), biologic excretion was assumed to be modeled as a series of monoexponential functions between each pair of time points with transitory washout rate λi starting from time point (ti). Cumulated activity was computed between each pair of time points as: Eq. 1for percentage retained whole-body γ-activity Rγ(ti). Each transitory decay constant λi was estimated as:
Eq. 1for percentage retained whole-body γ-activity Rγ(ti). Each transitory decay constant λi was estimated as: Eq. 2We assumed there was no further biologic excretion by the last time point, tfinal, so that terminal cumulated activity was estimated using physical radioactive decay only:
Eq. 2We assumed there was no further biologic excretion by the last time point, tfinal, so that terminal cumulated activity was estimated using physical radioactive decay only: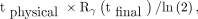 Eq. 3for 131I physical half-life tphysical = 8.03 d, and final whole-body counting percentage retention Rγ(tfinal) at the time of the final measurement. The sum of all cumulated activities yielded total cumulated activity, Aγ. The dose to blood in units of cGy/GBq of 131I, due to the γ-component (16), was:
Eq. 3for 131I physical half-life tphysical = 8.03 d, and final whole-body counting percentage retention Rγ(tfinal) at the time of the final measurement. The sum of all cumulated activities yielded total cumulated activity, Aγ. The dose to blood in units of cGy/GBq of 131I, due to the γ-component (16), was: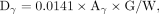 Eq. 4where G is an adjustment for body habitus (17), W is patient weight in kg, and 0.0141 is the S factor of dose to blood due to whole-body 131I γ-emissions (16).
Eq. 4where G is an adjustment for body habitus (17), W is patient weight in kg, and 0.0141 is the S factor of dose to blood due to whole-body 131I γ-emissions (16).
Blood sample data were handled in a manner similar to whole-body count measurements, except for the initial time, because blood was not drawn until 4 h after capsule ingestion. At time 0, percentage retention was estimated as: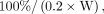 Eq. 5immediately after capsule ingestion, assuming patient blood volume is 20% of body weight (16). Blood sample well counter readings were calibrated to true activity using the 131I calibration capsule to convert counts into percentage retained activity. Cumulated activities of blood samples for emitted betas, Aβ, were computed similarly to the manner described for whole-body γ-camera counts, assuming excretion modeled as monoexponential functions between time points. Beyond the final blood measurement, the final additional number of emitted betas was estimated as:
Eq. 5immediately after capsule ingestion, assuming patient blood volume is 20% of body weight (16). Blood sample well counter readings were calibrated to true activity using the 131I calibration capsule to convert counts into percentage retained activity. Cumulated activities of blood samples for emitted betas, Aβ, were computed similarly to the manner described for whole-body γ-camera counts, assuming excretion modeled as monoexponential functions between time points. Beyond the final blood measurement, the final additional number of emitted betas was estimated as: Eq. 6for final blood sample percentage retention Rβ(tfinal). The dose to blood due to the β-component, Dβ, in units of cGy/GBq was:
Eq. 6for final blood sample percentage retention Rβ(tfinal). The dose to blood due to the β-component, Dβ, in units of cGy/GBq was: Eq. 7where 2.59 is the S factor for dose to blood caused by 131I β-emissions within the blood itself (16). Adding the 2 dose estimates Dγ and Dβ provided method 1 total radiation dose to blood-forming organs DTotal in units of cGy/GBq. Limiting the blood absorbed dose to 200 cGy yielded the MPA for method 1:
Eq. 7where 2.59 is the S factor for dose to blood caused by 131I β-emissions within the blood itself (16). Adding the 2 dose estimates Dγ and Dβ provided method 1 total radiation dose to blood-forming organs DTotal in units of cGy/GBq. Limiting the blood absorbed dose to 200 cGy yielded the MPA for method 1: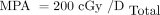 Eq. 8
Eq. 8
Method 2: Blood Measurements Only
Linear regression was used to define empirically the association between Dβ and DTotal by method 1, without reference to computed Dγ values. Linear regression intercept and slope predicted DTotal from blood measurements alone (method 2 dose). The MPA for method 2 was 200 cGy/method 2 dose.
Method 3: Whole-Body Camera Measurements Only
Linear regression was used to define empirically the association between Dγ and DTotal by method 1, without reference to computed Dβ values. Linear regression intercept and slope predicted DTotal based on γ-camera measurements alone (method 3 dose). The MPA for method 3 was 200 cGy/method 3 dose.
Method 4: 48-Hour Whole-Body Camera Measurements Only
Method 4 used whole-body γ-camera conjugate counts only for the 48-h imaging session (13), from which dose to blood-forming organs adjusted for patient height, weight, and blood volume (BLV) was computed as: Eq. 9where t48 was the time of the single imaging session in units of hours 48 h after ingestion of 131I capsule tracer activity, and BLV (mL) (18):
Eq. 9where t48 was the time of the single imaging session in units of hours 48 h after ingestion of 131I capsule tracer activity, and BLV (mL) (18): Eq. 10
Eq. 10 Eq. 11for height H in cm and weight W in kg. MPA for method 4 was 200 cGy/DTotal for DTotal by method 4 (Eq. 9).
Eq. 11for height H in cm and weight W in kg. MPA for method 4 was 200 cGy/DTotal for DTotal by method 4 (Eq. 9).
Statistical Analysis
Analyses were performed using commercially available software (Medcalc, version 7.5.0.0.; Medcalc Software, Inc.). Values are reported as mean ± SD. Continuous variables were assessed by the χ2 test to determine normality of distribution. The unpaired or paired t test, as appropriate, compared values between groups for continuous variables that were normally distributed; otherwise, the Wilcoxon test was used. Frequencies and percentages characterized categoric variables. χ2 analysis of proportions compared ratios. Linear regression tested correlations between continuous variables, and Bland–Altman analysis quantified trend and bias. The κ-statistic determined strength of agreement among methods 2–4 to identify cases for which method 1 determined MPA less than 7.4 GBq (19), and the McNemar test evaluated significance of differences among methods.
For all tests, a P value of less than 0.05 was defined as statistically significant.
RESULTS
DTotal Comparisons
Dβ contributed 70% ± 7% (47%–85%) to DTotal, and Dγ contributed 30% ± 7% (15%–53%). DTotal correlated with Dβ, with an r of 0.98. Method 2 total dose predicted from well counter measurements alone was: Eq. 12DTotal correlated with Dγ, with an r of 0.95. Method 3 total dose predicted from camera measurements alone was:
Eq. 12DTotal correlated with Dγ, with an r of 0.95. Method 3 total dose predicted from camera measurements alone was: Eq. 13Method 1 doses were not normally distributed (35.3, P < 0.0001). The blood dose predicted by method 2 was similar to method 1 (21.0 ± 13.7 cGy/GBq vs. 21.0 ± 14.1 cGy/GBq, P = 0.11), as was method 3 dose (20.9 ± 13.7 cGy/GBq, P = 0.73), but method 4 dose was significantly lower (16.3 ± 14.8 cGy/GBq, P < 0.0001). The mean percentage error versus method 1 dose was 0% ± 6%, 2% ± 11%, and −13% ± 21% for method 2, method 3, and method 4, respectively. Correlation with method 1 was significantly stronger for method 2 than for method 3 (r = 0.98 vs. r = 0.94, P = 0.001) and method 4 (r = 0.69, P < 0.0001) (Table 1). Bland–Altman limits of agreement were narrowest for method 2 (−5.6 to +5.6 cGy/GBq), wider for method 3, and widest for method 4 (−26.8 to +17.5 cGy/GBq) (Figs. 1–3).
Eq. 13Method 1 doses were not normally distributed (35.3, P < 0.0001). The blood dose predicted by method 2 was similar to method 1 (21.0 ± 13.7 cGy/GBq vs. 21.0 ± 14.1 cGy/GBq, P = 0.11), as was method 3 dose (20.9 ± 13.7 cGy/GBq, P = 0.73), but method 4 dose was significantly lower (16.3 ± 14.8 cGy/GBq, P < 0.0001). The mean percentage error versus method 1 dose was 0% ± 6%, 2% ± 11%, and −13% ± 21% for method 2, method 3, and method 4, respectively. Correlation with method 1 was significantly stronger for method 2 than for method 3 (r = 0.98 vs. r = 0.94, P = 0.001) and method 4 (r = 0.69, P < 0.0001) (Table 1). Bland–Altman limits of agreement were narrowest for method 2 (−5.6 to +5.6 cGy/GBq), wider for method 3, and widest for method 4 (−26.8 to +17.5 cGy/GBq) (Figs. 1–3).
Linear Regression and Bland–Altman Comparisons Versus Conventional Method 1 of Alternative Methods to Compute Total Blood Dose
Linear regression (A) and Bland–Altman plot (B) for method 2 vs. method 1 dose.
MPA Comparisons
Method 2 MPA was similar to method 1 MPA (14.4 ± 9.0 vs. 14.5 ± 9.3 GBq, P = 0.05), but MPA values were different from method 1 estimates for both method 3 (13.2 ± 6.7 GBq, P = 0.01) and method 4 (19.1 ± 11.1 GBq, P < 0.0001). Mean percentage error versus method 1 MPA was 0% ± 6%, −2% ± 11%, and 13% ± 21% for method 2, method 3, and method 4, respectively. Correlation with conventional method 1 MPA was strongest for method 2 (r = 0.99), less strong (P < 0.0001) for method 3 (r = 0.95), and least strong (P < 0.0001) for method 4 (r = 0.75) (Table 2). Bland–Altman analyses indicated slopes (trends) were not significant for method 2 but were for methods 3 and 4. Bland–Altman limits of agreement with method 1 was smallest for method 2 (−2.7 to +2.5 GBq), larger for method 3, and largest for method 4 (−9.8 to +19.0 GBq) (Figs. 4–6).
Linear Regression and Bland–Altman Comparisons Among Methods of MPA
Linear regression (A) and Bland–Altman plot (B) for method 3 vs. method 1 dose.
Linear regression (A) and Bland–Altman plot (B) for method 4 vs. method 1 dose.
Linear regression (A) and Bland–Altman plots (B) for method 2 MPA vs. method 1.
Linear regression (A) and Bland–Altman plots (B) for method 3 MPA vs. method 1.
Linear regression (A) and Bland-Altman plots (B) for method 4 MPA vs. method 1.
MPA Less Than 7.4 Gbq
Method 1 indicated that in 22 cases (31%), MPA should be less than 7.4 GBq (200 mCi), the empiric activity many institutions choose to administer (5). When method 1 was used as the reference standard, the κ-statistic indicated that best agreement with method 1 for identifying which patients should have an administered activity limited to less than 7.4 GBq was obtained for method 2, and weakest agreement was found for method 4 (Table 3) (19). Case categorization differences were not significant between methods 1 and 3, but were different for method 4 (12.1%, McNemar P = 0.02) (Table 3). Sensitivity to identify cases for which method 1 required an MPA of less than 7.4 GBq was higher for method 2 (20/22 cases) than for method 4 (10/22 cases) (91% vs. 45%, P = 0.004).
Comparison of Methods 2–4 Against Cases for Which MPA < 7.4 GBq by Conventional Method 1
Of the 2 cases missed by method 2, MPA was estimated to be 7.7 and 10.4 GBq by method 2 compared with 6.1 and 7.1 GBq by conventional method 1.
Previous Treatment and Abnormal Renal Function
Of the 71 studies, 32 (45%) had previous treatment (PT+) and 39 (55%) did not (PT−), whereas 23 studies (32%) had abnormal renal function (AF+) and 48 (68%) did not (AF−). Method 1 DTotal was different among these 4 patient groups (F ratio = 9.1, P < 0.001), as was conventional MPA (F ratio = 15.0, P < 0.001). Patients with abnormal renal function had higher DTotal (30.3 ± 10.2 vs. 16.5 ± 13.9 cGy/GBq, P = 0.0001) and lower MPA (7.4 ± 2.5 vs. 18.0 ± 9.4 GBq, P < 0.0001) than patients with normal renal function. Patients previously treated had lower DTotal (16.2 ± 12.7 vs. 45.9 ± 14.3 cGy/GBq, P = 0.01) and higher MPA (18.6 ± 10.2 vs. 11.2 ± 6.8 GBq, P = 0.001).
For all 71 sets of measurements, DTotal was similar for methods 1 and 2 (21 ± 14 vs. 21 ± 14 cGy/GBq, P = 0.97), as was MPA (14.5 ± 9.3 vs. 14.4 ± 9.0 GBq, P = 0.38). This was the case for each patient subgroup (Tables 4 and 5).
Comparison of Dose Estimates of Methods 2–4 Against Conventional Method 1 Dose Estimates for Patients Grouped by Prior Treatments and Abnormal Renal Function
Comparison of MPA of Methods 2–4 Against Conventional Method 1 MPA for Patients Grouped by Prior Treatments and Abnormal Renal Function
Correlation of method 2 to method 1 MPA was similar for PT−, PT+, AF−, and AF+ subgroups (r = 0.98, 0.99, 0.99, and 0.94, respectively). Camera-only, method 3, dose estimates differed from conventional estimates for the PT+ and AF− group, and method 3 MPA differed for both PT+ and AF− and PT− and AF+ groups. Forty-eight-hour camera-only, method 4, dose estimates differed from conventional values for PT− and AF− and PT− and AF− groups, and method 4 MPA differed for PT− and AF− and PT+ and AF+ groups (Tables 4 and 5).
DISCUSSION
Our main finding was that blood sampling alone suffices to estimate 131I MPA, even for patients with abnormal renal function.
The 2012 Society of Nuclear Medicine and Molecular Imaging guidelines indicate that 131I activity required for thyroid remnant ablation varies with the risk of distant metastases or recurrence of disease (14). In many institutions, a fixed empiric dose regimen is followed involving administration of 5.55–9.25 GBq (150–250 mCi) of 131I, but that approach can lead to undertreating some patients while overtreating others with a radiation dose greater than 200 cGy to blood, particularly in older patients (20). It is generally accepted that total dose to blood should be less than 200 cGy (21).
Optimizing 131I activity for treatment of thyroid cancer has received considerable attention in recent years (22,23). Some investigations have reported that higher success rates are obtained with higher administered activity (2.22–3.70 GBq compared with 1.11 GBq) (2,24). Aggressive protocols use repeated empiric activities of 11.1 GBq (300 mCi) at 3-mo intervals (25). Even higher activities (38.5 GBq of 131I) have been used; while successfully treating thyroid cancer, these protocols sometimes necessitate hospital admission for pancytopenia (26). Contravening these approaches is the precaution of limiting blood dose to less than 200 cGy to avoid leukopenia and thrombocytopenia (6,7). So, whereas higher doses are recommended for patients with metastatic disease, activities below 7.4 GBq are usually recommended to avoid serious complications affecting bone marrow (3,27).
Despite being the preferred approach, conventional MPA methods are time consuming, requiring as long as 7 d for prolonged iodine retention, motivating investigations to simplify these procedures. It is not surprising that method 2 using multiple blood samples over time agreed with the conventional method more closely than the method 4 single 48-h camera measurement. In contrast to blood samples that are processed on the same day under the same conditions, camera measurements performed over several days involve inherently more sources of variability, including patient positioning, and count variations due to different 131I activity distributions in various organ systems over time. For method 4 (13), variability is exacerbated by not knowing whether a single 48-h measurement is representative of 131I excretion over time. An alternative camera-only method predicting MPA from 48-h percentage retention by a nonlinear biexponential model reported an SD of 14.3% (28), similar to the 21% SD we found for method 4, and substantially higher than the 6% SD we found for method 2. With an SE of 10%, method 3’s camera measurements at multiple time points agreed more closely with conventional techniques than method 4’s use of a single time point, comparable to an earlier camera-only method that had an SE of 11% (12), but method 3 nonetheless showed a weaker correlation than method 2’s use of blood measurements alone.
One study found that 96-h blood sampling combined with 24-h or 48-h sampling yielded predictions closest to conventional MPA, but still required initial camera and blood measurements (29). In contrast, our method 2 uses no camera measurements. Our finding of lower MPA for patients with abnormal renal function agrees with reports of prolonged 131I excretion delivering high dose in these individuals (30). That method 2 agreed well with conventional values for patients with abnormal renal clearance was reassuring, because this group of patients is particularly concerning.
This study has several limitations. Our investigation addressed the narrow question of whether conventionally estimated 131I radiation dose can be predicted by blood measurements alone. Protocols have been proposed to quantify radiation dose by 131I tomography (31), but tomography was not part of our study, and there was no other independent reference standard available for definitively identifying intrinsic radiation dose delivered to our patients. A relevant question to answer is the degree to which specific radiation doses succeed in treating thyroid cancer. Therapy success rates are reported to correlate with dose to blood rather than to administered activity (32), whereas increasing activity to more than 5.55 GBq did not increase blood dose (3). So, whether patients actually have different outcomes by receiving an empiric amount of activity, such as all patients receiving 7.4 GBq, as opposed to after a dosing regimen based on estimating MPA, has yet to be proven. Conducting a prospective outcomes study of that nature would be difficult, for which satisfying ethical concerns would be challenging.
CONCLUSION
Because calculated MPA based on blood measurements alone is comparable to MPA obtained with combined body counting and blood sampling, blood measurements alone are sufficient for determining MPA, even in patients with metastatic thyroid carcinoma or compromised renal function.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Apr. 13, 2017.
- © 2017 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication February 27, 2017.
- Accepted for publication April 11, 2017.













