Abstract
Pretargeted radioimmunodiagnosis and radioimmunotherapy aim to efficiently combine antitumor antibodies and medicinal radioisotopes for high-contrast imaging and high–therapeutic-index (TI) tumor targeting, respectively. As opposed to conventional radioimmunoconjugates, pretargeted approaches separate the tumor-targeting step from the payload step, thereby amplifying tumor uptake while reducing normal-tissue exposure. Alongside contrast and TI, critical parameters include antibody immunogenicity and specificity, availability of radioisotopes, and ease of use in the clinic. Each of the steps can be optimized separately; as modular systems, they can find broad applications irrespective of tumor target, tumor type, or radioisotopes. Although this versatility presents enormous opportunity, pretargeting is complex and presents unique challenges for clinical translation and optimal use in patients. The purpose of this article is to provide a brief historical perspective on the origins and development of pretargeting strategies in nuclear medicine, emphasizing 2 protein delivery systems that have been extensively evaluated (i.e., biotin–streptavidin and hapten-bispecific monoclonal antibodies), as well as radiohaptens and radioisotopes. We also highlight recent innovations, including pretargeting with bioorthogonal chemistry and novel protein vectors (such as self-assembling and disassembling proteins and Affibody molecules). We caution the reader that this is by no means a comprehensive review of the past 3 decades of pretargeted radioimmunodiagnosis and pretargeted radioimmunotherapy. But we do aim to highlight major developmental milestones and to identify benchmarks for success with regard to TI and toxicity in preclinical models and clinically. We believe this approach will lead to the identification of key obstacles to clinical success, revive interest in the utility of radiotheranostics applications, and guide development of the next generation of pretargeted theranostics.
- general oncology
- radionuclide therapy
- radiopharmaceuticals
- multistep
- pretargeted radioimmunotherapy
- radioimmunotherapy
Monoclonal antibodies (mAbs) are attractive vehicles for delivering cytotoxic payloads to tumors using cell-surface targets as ZIP codes. However, the complexity of the tumor microenvironment and the pharmacokinetics of mAbs in vivo have created major hurdles in radioimmunodiagnosis and radioimmunotherapy (1). Radioimmunoconjugates have been approved by the U.S. Food and Drug Administration for clinical oncology use (4 imaging agents, beginning with OncoScint [Cytogen Corp.] for imaging of tumor-associated glycoprotein 72 [TAG-72] in 1992 and Zevalin [Acrotech Biopharma] and Bexxar [GlaxoSmithKline] for therapy targeting CD20 in the early 2000s). However, clinical use (and hence, commercial success) has been hampered by unanticipated physician preferences (2) and numerous challenges universal to radiopharmaceutical therapies (3).
Despite the success in treating radiosensitive hematologic malignancies, radioimmunotherapy in the treatment of solid tumors has been clinically unsuccessful (1). Nevertheless, a recent revision of the radiobiologic paradigms of targeted α-therapy has revealed a highly complex response cascade comprising direct, bystander, and systemic effects (4), proving remarkably effective against β-refractive and bulky disease (5). Coupled with an enhanced development and application of theranostics in nuclear medicine, this response cascade has fueled development of a new generation of mAb theranostics against solid-tumor antigens, particularly with α-emitting isotopes (e.g., City of Hope’s anti–carcinoembryonic antigen [CEA] 225Ac-DOTA-5MA NCT05204147; Bayer’s suite of 227Th-IgG drugs targeting mesothelin, prostate-specific membrane antigen, or human epidermal growth factor receptor 2 [HER2] antigens NCT03507452, NCT03724747, and NCT04147819; Janssen’s 225Ac-DOTA-h11B6 targeting human kallikrein 2 NCT04644770; and Fusion’s 225Ac-FPI-1434 targeting insulinlike growth factor type 1 receptor). More details can be found in several excellent reviews (6–9).
The field waits with great interest to see the outcome of these radioimmunotherapy clinical trials of directly labeled mAbs. Nonetheless, based on prior experience with other radioimmunotherapeutics, there is concern that hematopoietic toxicity will prove to be dose-limiting, a common hurdle for molecularly targeted radiopharmaceuticals (3,10). Fundamentally, the necessary therapeutic indices (TIs, or tumor–to–normal-tissue absorbed dose ratios) for meaningful radioimmunotherapy of solid-tumor masses were not achieved, partly because of inadequate mAb uptake (subtherapeutic radiation dose for solid tumors), dose-limiting toxicities (DLTs) because of poor TIs (causing myelotoxicity and renal toxicity), and antidrug antibodies (ADAs) (1). The radiopharmacology of radioimmunoconjugates has so far permitted mostly suboptimal TIs, especially for critical radiosensitive tissues such as bone marrow and kidney. Current and future advances in protein engineering and radioligand chemistry are necessary to overcome these barriers.
Pretargeted radioimmunodiagnosis (PRID) and pretargeted radioimmunotherapy (PRIT) separate the tumor targeting and the radiocarrier (e.g., a radiohapten) delivery steps, vastly improving the contrast and TIs while creating modular systems with individual optimization ease (Fig. 1). The tumor-targeting bispecific protein can be engineered to improve tumor uptake and TI; a chase molecule or clearing agent (CA) can be designed to sequester unbound proteins from blood to liver for metabolism. After an optimal pretargeting interval of hours or days, an intravenous payload with high affinity for the second specificity in the protein seeks out the bispecific protein targeted to the tumor or clears from the body in minutes to hours.
Comparison of conventional radioimmunotherapy and PRID/PRIT and compatible vector survey. (A) Injection of radioimmunoconjugate (left) leads to low TIs, especially in hematopoietic and highly perfused tissues. With 3-step BsAb pretargeting (middle), BsAb is administered, followed 1 d later by CA to quickly reduce circulating BsAb. During final step, administered radiocarrier (e.g., radiohapten) is captured by intratumoral BsAb or rapidly cleared. A 2-step approach (right) is feasible with SADA BsAb innovation. (Adapted from (153)). (B) Select bispecific antitumor/antiradiocarrier vectors.
Current PRID and PRIT approaches harness advances in protein engineering and bioorthogonal chemistry to overcome the limitations of previous PRID and PRIT systems. However, PRID and PRIT present additional complexity in terms of drug manufacture (developing at least 2 products, and perhaps a CA) and dosing protocol optimization (both dose and pretargeting interval). Here, we provide a historical perspective of PRID and PRIT and explore potential ways by which PRID and PRIT can be further optimized to deliver a high radiation dose to tumor while improving TIs substantially for critical radiosensitive tissues.
HISTORICAL PERSPECTIVE
Concept of PRID and PRIT
Building on initial investigations of in vivo tumor targeting with radiolabeled polyclonal antibodies (11,12) and fueled by the discovery of mAbs by Köhler and Milstein (13), radioimmunodiagnosis using mAbs surged in the late 1970s, and radioimmunotherapy soon followed (14,15). However, despite the antigen specificity of mAbs, most did not translate into high-contrast tumor imaging (15). The critical hurdle—that is, disappointingly low overall tumor uptake and high normal-tissue background with radiolabeled IgG mAbs—was realized early on, requiring 99mTc blood-pool agents for computer subtraction from 131I-mAb images (16).
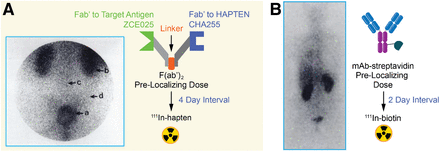
In the mid 1980s, Goodwin et al. pioneered one of the first examples of pretargeting of radioisotopes by engineering metal chelate–specific mAbs (17). This example was predicated on the physicochemical properties of metal chelate complexes (often low-molecular-weight and hydrophilic combined with net negative charge and high kinetic stability under physiologic conditions) possessing extremely favorable in vivo pharmacokinetics and biodistribution. The antichelate mAb CHA255 passively entered tumors and, after a pretargeting interval of 24 h, was chased with a metal chelate hapten, indium (III)-4-[N′-(2-hydroxyethyl)thioureido]-l-benzyl-ethylenediaminetetraacetic acid (18). The concept of chelate chase—that is, using pharmacologic doses of an empty nonradioactive chelate hapten to force rapid renal excretion—significantly enhanced contrast, thereby reducing normal-organ radiation exposure by as much as 95% (18,19). Soon after, Stickney et al. conducted the first clinical trial of PRID in patients with CEA-expressing colorectal cancer (CRC) using a bispecific antibody (BsAb) anti-CEA/anti–metal chelate hapten system (20). Besides establishing the feasibility of the approach in this study of 14 patients, 20 of 21 known lesions were detected, for an overall sensitivity of 95%; 8 of 9 new lesions were confirmed; and high contrast was observed at as early as 4 h (Fig. 2A) (20).
Initial PRID studies of tumors in patients. (A) BsAb pretargeting system. Planar posterior pelvic scan (coronal view) of patient with recurrent CRC invading right sacral area was obtained 3 d after injection of 111In-hapten. a =tumor; b =kidneys; c =spine; d = iliac crest. (Reprinted with permission of (20).) (B) Biotin–streptavidin pretargeting system. Anterior chest and upper abdomen image (coronal view) of patient with recurrent squamous cell carcinoma of right lung was obtained 2 h after injection of 111In-biotin. a = tumor; b = kidneys; c = bladder. (Reprinted from (26).)
A Surge in PRID—Especially Driven by Biotin–Streptavidin Approaches—and the Potential of PRIT
Although PRID with BsAb showed considerable promise, issues related to TIs as a consequence of insufficient affinity or avidity for the hapten (e.g., low-nanomolar range) prompted the development of alternatives. Streptavidin (53 kDa protein from the bacterium Streptomyces avidinii) and avidin (66 kDa protein found in egg whites) are both tetrameric proteins, with each individual subunit able to bind a single molecule of biotin (244 Da) with similar affinities (femtomolar). With these significantly higher affinities, the biotin–streptavidin system was an attractive candidate for pretargeting.
One of the first pretargeting applications of the biotin–streptavidin system was devised by Hnatowich et al. in 1987, consisting of an antitumor mAb–streptavidin conjugate, a chase molecule, and radiolabeled biotin (21). Afterward (1987–1988), multiple research groups explored various biotin–streptavidin reagents, establishing the immunoreactivity and in vivo fate of biotinylated or mAb–streptavidin pharmacokinetics, clearance of radiolabeled forms of biotin and streptavidin, and safety in animals (22,23). Notably, Pimm et al. documented that radioiodinated avidin and streptavidin were cleared from the circulation via the kidneys, but with prolonged renal retention (22). In addition to issues of immunogenicity, this renal retention of streptavidin (and hence low TI for the kidney) became problematic and dose-limiting, prompting efforts at alleviation via chemical modification (e.g., with succinic anhydride or 1,2-cyclohexanedione) or engineering of recombinant streptavidin (24,25). In 1990, Kalofonos et al. reported the clinical feasibility of biotin–streptavidin PRID (26). In 10 patients with squamous cell carcinoma of the lung, the investigators infused antihuman milk fat globule antigen 1 mAb–streptavidin for tumor targeting, followed 2–3 d later with 111In-biotin (26). Positive imaging was observed in 8 of 10 lung tumors (Fig. 2B) (26).
In 1991, Paganelli et al. conducted their first biotin–streptavidin PRID clinical study on 19 patients with a confirmed histologic diagnosis of tumors using an optimized 3-step regimen consisting of biotinylated anti-CEA mAb, an avidin chase, and 111In-biotin (27). Tumors and metastases were detected in 18 of 19 patients (the remaining patient was a true-negative) less than 3 h after administration of 111In-biotin by γ-scintigraphy, with no evidence of toxicity and with favorable contrast (27).
Although PRID showed promise, its development was soon blunted by the advent of oncologic 18F-FDG PET/CT (28). Also, for diagnosis, sufficient image contrast and a single-day imaging procedure was achievable with rapidly clearing antibody-based probes. Unlike radioimmunodiagnosis, for which high tumor–to–normal-tissue contrast is necessary at only 1 specific time point, radioimmunotherapy requires high contrast at all time points in order to achieve high TIs. PRIT soon became the major platform to further the science and its clinical translation. The primary consideration for PRID is its theranostic potential: directing the surgical procedure to sites of the body with a high likelihood of finding antigen-bearing tumor, and performing dosimetry using quantifiable radioisotopes, a critical predictor of tumor response to PRIT. Tables 1 and 2 summarize the clinical and preclinical PRIT studies, respectively, with an emphasis on dosimetry and TIs described here.
Select Clinical PRIT and Targeted Radiotherapy Studies with Emphasis on Dosimetry
Select Preclinical PRIT and Targeted Radiotherapy Studies with Emphasis on Dosimetry
Published in 1997, pilot clinical PRIT studies by Paganelli et al., using 111In/90Y-biotin targeting against various tumor types with anti-CEA, anti-TAG-72, and antitenascin mAbs (29), demonstrated that favorable TIs could be achieved. Mean absorbed doses (as cGy/37 MBq) to tumor, marrow, kidney, and liver were 15.2 ± 8.7, 1.1 ± 0.6 (TI, 13.8), 4.5 ± 2.1 (TI, 3.4), and 1.5 ± 1.0 (TI, 10.1), respectively. Eleven patients with various tumors (breast, brain, colon) were administered 90Y-biotin doses ranging from 1.85 to 5.55 GBq; the treatment was well tolerated, and no acute toxicity was observed. However, ADA to streptavidin was observed in all patients.
Over the next decade, Paganelli et al. studied multiple biotin–streptavidin PRIT dosing protocols and injection routes (reviewed by Papi et al. (30)). Remarkably, from 1994 to 2005, over 500 glioblastoma patients were treated with biotin–streptavidin PRIT. For example, during a phase I/II study (31), they treated 48 patients (possessing histologically confirmed grade III and IV gliomas) using 5-step intravenous antitenascin PRIT with 111In/90Y-biotin (90Y-biotin dose range, 2.22–2.96 GBq/m2). The DLT was hematologic, and the maximum tolerated dose (MTD) was 2.96 GBq/m2. They reported tumor, brain, kidney, liver, and marrow mean absorbed doses (as cGy/37 MBq) of 15.20 ± 8.70, 0.6 ± 0.3 (TI, 25.3), 2.7 ± 1.6 (TI, 5.63), 1.5 ± 1.0 (TI, 10.1), and 0.8 ± 0.5 (TI, 19.0), respectively. ADA to streptavidin was observed in all patients.
These early clinical PRIT studies inspired many groups to explore biotin–streptavidin PRIT in the mid to late 1990s (32,33). Much effort was devoted to improving BsAb design and alternative second specificities (besides biotin or metal chelator haptens), including complementary oligomeric pretargeting systems (34–36). Using morpholino complementary oligomers, Liu et al. and Hnatowich et al. have shown success for in vivo applications (reviewed by Liu (37)), although they have not been investigated clinically.
NeoRx Corporation exploited mAb–streptavidin fusion proteins to pretarget tumors before administration of radiobiotin. During the early to mid 1990s, they developed a mAb–streptavidin chemical conjugate (mAb NR-LU-10 specific for epithelial cell adhesion molecule) to target multiple solid-tumor types (38). In 2000, they showed how a single treatment with pretargeted 90Y-biotin was curative in nude mice bearing human tumor xenografts (CRC, lung, and breast) without significant toxicity (39). Although TIs were not reported, an approximately 20-fold improvement in the tumor-to-blood area under the curve (AUC) ratio (calculated from 0 to 120 h after injection) for PRIT versus conventional radioimmunotherapy was demonstrated (tumor-to-blood AUC ratio: 28.3 and 1.22, respectively). At doses of up to 29.6 MBq (MTD not reached) of pretargeted 90Y-biotin per mouse, 28 of 30 cures were achieved with transient myelosuppression and no apparent sequelae. Moreover, kidney uptake and retention were relatively low, at approximately 2% of the injected dose per gram from 2 to 120 h after injection. During a phase I dose escalation study (40), the MTD was determined to be 4.07 GBq/m2, the DLT at 5.18 GBq/m2 was gastrointestinal toxicity, and the recommended phase 2 dose was 4.44 GBq/m2. These findings were considered highly remarkable because DLT was not hematologic and 90Y doses approximately 5 times higher than radioimmunotherapy could be administered without marrow DLT or use of stem cell support (41). Imaging and dosimetry studies (42) revealed tumor, small-intestine, large-intestine, kidney, liver, and marrow mean absorbed doses (as cGy/37 MBq) of 16.4 ± 13.2, 49.2 ± 25.3 (TI, 0.33), 34.8 ± 17.9 (TI, 0.47), 11.5 ± 5.6 (TI, 1.43), 3.0 ± 1.8 (TI, 5.47), and 0.34 ± 0.08 (TI, 48.2), respectively. High doses of 90Y-biotin in the gastrointestinal tract resulted from cross-reactivity of the mAb NR-LU-10 with the bowel epithelium. Moreover, tumor response was seen in the 2 patients with the highest estimated dose to tumor (4,000–6,000 cGy); grade IV diarrhea was observed in patients estimated to have received 6,850–14,000 cGy to the large-intestine wall; and delayed renal toxicity was observed in patients estimated to have received 2,170 or 3,072 cGy. In the same year, Knox et al. reported disappointing results from a phase II clinical study on 25 patients with metastatic CRC after a single dose of mAb–streptavidin pretargeted 90Y-biotin, 4.07 GBq/m2 (mean administered dose, 3.941 ± 0.381 GBq/m2) (43). The overall response rate was modest (8%), and both hematologic and nonhematologic toxicities were observed (severe diarrhea in 30% of patients and delayed renal toxicity in 2 patients).
NeoRx moved on to an alternative pan-carcinoma mAb/antigen system, in which a mAb–streptavidin chemical conjugate (mAb CC49 specific for TAG-72) was used to deliver radiobiotin (44). Anti-TAG-72 radioimmunotherapy was studied clinically in the mid 1990s; for example, 111In/90Y-CC49 was evaluated in 12 patients with metastatic gastrointestinal tract carcinomas (45). Preclinical pretargeting studies on nude mice bearing human tumor xenografts were promising, as the tumor-to-blood AUC ratios were 179, 170, and 371 for 149Pm-, 166Ho-, and 177Lu-biotin, respectively (no TIs were reported), and kidney uptake was minimal, at approximately 1%–2% of the injected dose per gram from 1 to 168 h after injection (44). In 2005, a phase I 3-step PRIT trial was performed using a CC49-(single-chain variable fragment [scFv])4–streptavidin fusion (46). A total of 9 advanced-CRC patients received CC49-(scFv)4–streptavidin, CA, and 111In/90Y-biotin (90Y-biotin dose of 0.37 GBq/m2). Imaging and dosimetry studies revealed a patient-specific mean 90Y radiation dose (as cGy/37 MBq) of 7.02 (range, 3.36–11.2) to kidneys, 3.75 (range, 0.63–6.89) to liver, 0.22 (range, 0.12–0.34) to marrow, and 28.9 (range, 4.18–121.6) to tumors, corresponding to TIs of 4.12, 7.71, and 131 for kidney, liver, and marrow, respectively (46). MTD, DLT, and recommended phase 2 dose were not defined; however, the low TI for kidney was projected to be dose-limiting. Förster et al. demonstrated that succinylation of the CC49-(scFv)4–streptavidin construct could reduce kidney uptake (47), but this was never tested clinically.
Additional solid-tumor mAb/antigen systems studied by NeoRx for PRIT included Lewis Y antigen (with 213Bi-biotin (48) or 90Y-biotin (49)) and mesothelin (with 177Lu-biotin or 90Y-biotin (50)). Also, using a novel antidisialoganglioside (GD2)-(scFv)4–streptavidin fusion protein, highly efficient GD2 targeting was demonstrated with 111In-biotin in nude mice bearing human tumor xenografts (51). Tumor-absorbed radiation doses (as cGy/37 MBq) were 8,784 ± 1,495 and 5,665 ± 307 for conventional radioimmunotherapy and PRIT with 90Y-biotin, respectively (51). For radioimmunotherapy, TIs of 3, 13, and 19 were determined for blood, kidney, and liver, respectively. In comparison, for PRIT with 90Y-biotin, TI was improved for blood (TI, 170) but diminished for kidney (TI, 3) and similar for liver (TI, 21). Although highly promising in terms of reducing myelotoxicity, the poor TI for kidney limited its translational potential.
NeoRx also developed reagents for PRIT of hematologic cancers. In the mid 1990s, remarkably, anti-CD20 radioimmunotherapy was shown to be curative in patients with relapsed B-cell lymphomas; however, highly aggressive myeloablative treatments with bone marrow rescue were needed (52). In 2002, the results from the anti-CD20 radioimmunotherapy phase 3 study of 90Y-ibritumomab were reported, showing that treatment was well tolerated and superior to rituximab in terms of overall response rate and complete response rate (53). NeoRx collaborated with Press et al. at the Fred Hutchinson Cancer Research Center and the University of Washington to develop anti-CD20 biotin–streptavidin PRIT to improve the safety profile of anti-CD20 radioimmunotherapy (54). They prepared an anti-CD20–streptavidin chemical conjugate for PRIT with 90Y-biotin and performed preclinical studies on nude mice bearing human Ramos xenografts, making direct comparisons with radioimmunotherapy (54). Although no TIs were reported, tumor-to-blood ratios at 24 h were markedly improved with PRIT (3 and 0.4 for PRIT and radioimmunotherapy, respectively). Notably, superior tumor uptake was also shown with PRIT, establishing the potential advantage of PRIT particularly for noninternalizing antibodies. A lethal dose of 14.8 MBq was reported for radioimmunotherapy, with all treated animals dying of marrow suppression and infection on day 10. In contrast, 9 of 9 mice receiving pretargeted 90Y-biotin (29.6 MBq) achieved CRs by day 12, leading to 8 of 9 cures (no recurrences during observation period of >140 d) and minimal toxicity.
These highly promising preclinical PRIT studies led to a phase I/II study on non-Hodgkin lymphoma patients (41). Seven patients with relapsed or refractory non-Hodgkin lymphoma received 111In/90Y-biotin (90Y-biotin dose of 1.11 or 1.85 GBq/m2). Tumor, kidney, liver, and marrow mean absorbed doses (as cGy/37 MBq) were 29 ± 23, 5.0 ± 1.7 (TI, 5.8), 1.6 ± 0.5 (TI, 18), and 0.16 ± 0.1 (TI, 181), respectively; also, the estimate of tumor–to–whole-body dose ratio (38:1) achieved with PRIT was higher than has been achieved using conventional radioimmunotherapy. Furthermore, doses of 90Y 3 times the MTD of radioimmunotherapy could be given without significant myelosuppression. As a result, 6 of 7 achieved objective tumor regression, including 3 complete response and 1 partial response (41). Only grade I/II nonhematologic toxicity was observed, and grade III hematologic toxicity was transient in 5 of 7 patients. Six of 10 patients developed measurable ADA. MTD was not defined but was noted to be “likely more than 1.85 GBq/m2” on the basis of dose-limiting hematologic toxicity, and kidneys showed the highest uptake (5.0 ± 1.7 cGy/37 MBq vs. 1.3 cGy/37 MBq for unbound radiobiotin (55)).
To generate well-defined and homogeneous fusion proteins and greatly simplify manufacturing, the group developed a second-generation, genetically engineered anti-CD20–streptavidin fusion protein (as a (scFv)4–streptavidin fusion, B9E9FP (56)), documented a tumor-to-blood AUC ratio of more than 60 in nude mice bearing Ramos xenografts, and performed a phase I pilot trial in B-cell non-Hodgkin lymphoma (57). Fifteen non-Hodgkin lymphoma patients received B9E9FP, CA, and 111In/90Y-biotin (90Y-biotin dose, 0.555 GBq/m2). Mean absorbed doses to tumor, kidney, liver, and marrow (as cGy/37 MBq) were 26 ± 4, 7.7 ± 1.7 (TI, 3.4), 1.2 ± 0.2 (TI, 22), and 0.25 ± 0.04 (TI, 104), respectively. MTD, DLT, and recommended phase 2 dose were not defined. ADA was substantial 3 patients, and 5 patients had transient low antibody responses.
Additional hematologic tumor mAb/antigen systems studied by NeoRx for PRIT included CD25 (with 90Y-biotin or 213Bi-biotin (58)) and CD45 (with 90Y-biotin (59)). In a notable study, Pantelias et al. (60) evaluated multiantigen PRID with mAb–streptavidin (anti-CD20, anti-human leukocyte antigen DR, anti-CD22) and 111In-biotin. Interestingly, the most favorable tumor–to–normal-organ ratios of absorbed radioactivity were obtained using single conjugates optimized for target tumor antigen expression rather than the combination therapy (60). Of these additional PRIT systems, CD45 has been evaluated clinically.
Second-Generation BsAb PRIT with Multivalent Haptens
Alongside biotin–streptavidin PRIT development in the late 1980s, groups were also looking to optimize contrast by improving hapten selectivity for intratumoral BsAb over circulating BsAb. In 1989, Le Doussal et al. (Immunotech) evaluated PRID with bivalent hapten tracers to image CEA-expressing CRC (61). Coined “affinity enhancement,” they showed greater affinity to cell-bound than to unbound BsAb via cooperative cross-linking of intratumoral BsAb, resulting in additional tumor-absorbed dose (61). Also, a chase or CA was not required for high contrast, greatly simplifying the pretargeting regimen. Around the same time, Goodwin et al. reported a bivalent Janus hapten for pretargeting with their antichelate mAbs (62).
Between 1993 and 1998, Immunotech performed clinical PRID studies with an anti-CEA mAb/metal chelate–specific mAb BsAb. The metal chelate-specific mAb was against indium-diethylenetriaminepentaacetic acid (indium-DTPA). With a bivalent 111In-diDPTA-tyrosyl-lysine hapten for PRID, they demonstrated that high-contrast images could be obtained in patients with CRC (63), medullary thyroid cancer (MTC) (64,65), and small-cell lung cancer (66). Although the images were impressive, the use of murine BsAb led to ADA in most patients (e.g., ∼60% (63)). Furthermore, for PRIT with 131I, instead of developing a new metal chelate–specific mAb, they used stable indium-DTPA as an affinity handle to generate 131I-labeled diDTPA(In)-tyrosyl-lysine radiohapten (131I-diDTPA(indium)-hapten) and analogs for chelation of 99mTc and 188Re (67–69). In nude mice bearing human MTC xenografts, pretargeting of 92.5 MBq of 131I-diDTPA(indium)-hapten was demonstrated to be more efficient (leading to significantly longer growth delays) and less toxic than radioimmunotherapy (70). Soon after, this approach was evaluated in patients.
Initially, 2 phase I/II clinical trials assessing PRIT with 131I-diDTPA(indium)-hapten were performed on patients with either MTC (71) or small-cell lung cancer (72). The BsAb was a F(ab)′ × F(ab)′ chemical conjugate. Twenty-six MTC patients received 1–3 treatments at 131I-diDTPA(indium)-hapten doses ranging from 0.888 to 2.22 GBq/m2 (71). Tumor, kidney, liver, and marrow mean absorbed doses (as cGy/37 MBq) were 44.33 ± 53.39, 5.61 ± 2.02 (TI, 8.36 ± 10.02), 5.19 ± 2.23 (TI, 10.86 ± 13.55), and 1.60 ± 0.82 (TI, 29.62 ± 35.32), respectively (71). Myelosuppression was the crucial factor for DLT, and MTD (and recommended phase 2 dose) was 1.78 GBq/m2 (71). Among the 17 evaluable patients, 5 minor tumor responses were observed in patients with mainly a small tumor burden (71). ADA was observed in 9 of 17 (53%) patients (71). During the second clinical trial, 14 patients with small-cell lung cancer were treated with 131I-diDTPA(indium)-hapten ranging from 1.48 to 6.66 GBq (72). Tumor, kidney, liver, and marrow absorbed doses (as cGy/37 MBq) were 1.8–32.2, 3.9–5.0, 1.6–5.0, and 0.4–1.7, respectively. MTD without hematologic rescue was 5.55 GBq (72). Of the 12 patients, 2 partial response and 1 stabilization of more than 24 mo was observed (72). Although no recommended phase 2 dose was specified, dose escalation was reported to be continuing to reach 11.1 GBq (72). Among 5 evaluable patients for ADA, 1 patient showed significant ADA after 2 mo, which persisted at 12 mo (72).
A subsequent clinical trial was performed to optimize reagent dosing and timing of administration with humanized BsAb in 35 patients with CEA-expressing tumors (73). BsAb doses ranged from 10 to 100 mg/m2, 131I-diDTPA(indium)-hapten doses ranged from 1.9 to 5.5 GBq, and a pretargeting interval of 5 or 7 d was studied (73). With optimized PRIT (e.g., with 40 mg/m2 of BsAb and a 5-d pretargeting interval), 131I-diDTPA(indium)-hapten doses of up to 5.5 GBq were well tolerated in the absence of bone marrow involvement (73).
In a follow-up trial on 22 patients with CEA-expressing tumors, the humanized BsAb dose (40–75 mg/m2) and 131I-diDTPA(indium)-hapten dose (1.8–2.9 GBq/m2; 1.9–5.5 GBq) were varied to evaluate antitumor efficacy and toxicity (74). Myelosuppression was BsAb dose–dependent, with 75 mg/m2 leading to high hematologic toxicity, and nonhematologic toxicity was hepatic (transient grade I or II) (74). With a BsAb dose of 75 mg/m2, higher whole-body and liver mean radiation doses were observed (0.38 and 1.9 Gy for whole body and liver, respectively) than for 40 mg/m2 (0.33 and 1.4 Gy for whole body and liver, respectively), and mean tumor doses did not differ significantly with BsAb dose (75 mg/m2, 10.7 Gy [range, 1.7–53.5 Gy]; 40 mg/m2, 18.5 Gy [range, 2.4–49.3 Gy]) (74). Modest therapeutic efficacy was reported, with no CRs or PRs (74). The MTD was determined to be 3 GBq of 131I-diDTPA(indium)-hapten in MTC patients and was not defined in non-MTC patients (escalated beyond 5.5 GBq) (74). Human antimouse antibody elevation was observed in 1 patient (8%), and human antihuman antibody was observed in 4 patients (33%) (74).
In 2006, Chatal et al. reviewed their clinical experience and compared the survival of advanced-MTC patients who underwent PRIT (131I-diDTPA(indium)-hapten doses ranging from 1.9 to 5.5 GBq) with that of contemporaneous untreated patients for whom data were collected by the French Endocrine Tumor Group (75). Notably, they showed a survival benefit for those treated with PRIT, underscoring its clinical promise (75).
Besides CEA-expressing tumors, a PRID approach to imaging renal cell carcinoma was developed with bivalent haptens. Use of an anti–renal cell carcinoma/anti–indium-DTPA hapten BsAb and a refined tetrapeptide bivalent hapten, 111In-diDTPA-Phe-Lys-Tyr-Lys (111In-diDTPA-FKYK), showed highly efficient tumor targeting (76,77). During comparative studies with monovalent 111In-DTPA and 111In-diDTPA-FKYK, they achieved dramatic improvements in tumor uptake with the bivalent hapten (in nude mice bearing human renal cell carcinoma xenografts: ∼78% vs. ∼2% of the injected dose per gram at 4 h after injection for the bivalent and monovalent haptens, respectively) without sacrificing promising tumor-to-blood ratios (76). The 111In-diDTPA-FKYK was also prepared with d-amino acids to make it more resistant to in vivo peptidases and improve the residualization of 125I during PRIT (78). Efficient targeting of CEA was also demonstrated via this approach (with 111In, 99mTc, nonresidualizing 125I, or residualizing 125I) (79); however, antichelate mAbs were falling out of favor compared with alternative antihapten mAbs.
Third-Generation BsAb PRIT with Anti–Histidine-Succinyl-Glycine (HSG) mAb and BsAb Prepared via Dock-and-Lock (DNL)
The anti–111In-DTPA hapten approach was limited by mAb specificity and could not be used to target 90Y or 177Lu (80). Although additional antichelate mAbs were prepared (e.g., anti--copper-triethylenetetramine and anti–yttrium-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (yttrium-DOTA) (81)), alternative antihapten mAbs were evaluated for PRIT, including anti-2,4-dinitrophenyl (61) and anti-HSG pseudopeptide (82). Also, around this time, novel BsAb formats with divalent tumor-antigen binding (e.g., anti-CEA IgG or anti-CEA F(ab′)2 chemically conjugated to anti–indium-DTPA Fab′) were studied (83). Notably, pretargeting with anti-CEA IgG × anti–indium-DTPA Fab′ led to the most favorable tumor uptake and retention in the tumor, but use of a CA was necessary to achieve acceptable tumor-to-blood ratios (83). Therefore, a F(ab′)2–Fab′ BsAb (∼80 kDa) was considered optimum in terms of balancing tumor uptake and clearance without the need for a CA step (83).
In 2003, Sharkey et al. significantly advanced the HSG system for PRIT by developing a novel BsAb (e.g., anti-CEA F(ab′)2 or anticolon-specific antigen-p F(ab′)2 chemically conjugated to anti-HSG Fab) and a suite of HSG peptides suitable for targeting a variety of clinically relevant radionuclides (IMP241 for 90Y, 111In, and 177Lu, and IMP245 for 99mTc and 188Re) (84). Dosimetry projections for pretargeting anticolon-specific antigen-p with 90Y- or 177Lu-IMP241 in nude mice bearing human xenografts were 5,587–14,356 cGy/37 MBq for tumor, with corresponding TIs of 35–45, 8–9, and 26–35 for blood, kidney, and liver, respectively (84). In 2005, they clearly demonstrated the advantage of this pretargeting approach over a clinically used 99mTc-labeled CEA-specific F(ab′) for CRC imaging in mice bearing human xenografts (85). In 2006, an alternative peptide scaffold to IMP241 with less kidney retention was developed. IMP288 and PRID with 124I was reported (86).
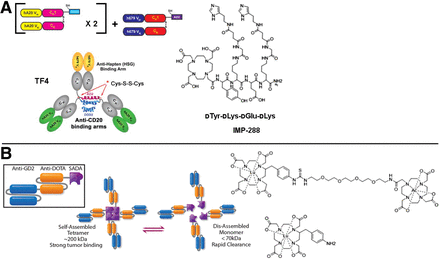
As was done for mAb–streptavidin fusions for biotin–streptavidin pretargeting, recombinant antitumor/antihapten BsAbs were replacing chemical conjugates. In 2003, Rossi et al. described a trivalent BsAb (hBS14: bivalent CEA and monovalent HSG); however, the engineering approach using transgenic myeloma cells led to low expression yield (87). Soon after, they described the DNL approach to assemble a multivalent tri-Fab antitumor/anti-HSG hapten BsAb with a molecular weight of approximately 157 kDa by exploiting regulatory protein kinase A dimerization and docking domains, and the anchoring domain of an interactive A-kinase anchoring protein, to form a stably tethered complex (Fig. 3A) (88,89). Interestingly, the blood clearance of the DNL BsAb was much faster than that of IgG (∼150 kDa) since the BsAb lacks the CH2 domain to enable neonatal Fc receptor recycling. DNL BsAbs have been generated against a variety of tumor antigens, including CEA (TF2), CD20 (TF4), and trophoblast cell surface antigen 2 (TF12); detailed reviews have been published (28,90). Dosimetry results for IMP325 (i.e., IMP288 saturated with nonradioactive indium) pretargeted to CEA-expressing LS-174T human CRC subcutaneous tumors were quite favorable; for example, for pretargeting of 177Lu-IMP325, the authors reported TIs of 169 and 7 for blood and kidney, respectively, with an estimated tumor-absorbed dose of 2,695 cGy/37 MBq (91).
BsAb pretargeting with DNL BsAb or with SADA BsAb platform. (A) Structure of tri-Fab TF4 made by DNL method and associated HSG hapten, IMP-288. DNL BsAb has single binding site for hapten and 2 binding sites for tumor antigen. (Reprinted with permission of (154).) (B) Structure of anti-GD2/anti-DOTA SADA BsAb and 225Ac-DOTA-hapten (reprinted from (123)) and 177Lu-aminobenzyl-DOTA (reprinted with permission of (127)). SADA BsAb has 4 binding sites for both DOTA hapten and tumor antigen. hA20 = humanized anti-CD20 IgG hA20 (veltuzumab); VH = heavy chain variable domain; VK = light chain variable domain; CH1 = heavy-chain constant domain 1; CK = light-chain constant domain; DDD2 = dimerization and docking domain with SEQ ID NO: 2; AD2 = anchoring domain with SEQ ID NO: 4.
In one notable DNL PRIT preclinical study, they prepared anti-PAM4-antigen BsAb TF10 for targeting of 90Y-IMP288 (92). They demonstrated in nude mice bearing established Capan-1 human pancreatic cancer xenografts that PRIT could be safely combined with gemcitabine and CRs could be achieved (92). Furthermore, they showed that doses of up to 33.3 MBq of pretargeted 90Y-IMP288 were well tolerated over 9 mo with no evidence of chronic nephrotoxicity (MTD not reached), a marked improvement from initial 90Y-IMP288 PRIT studies (93).
Initial clinical experience with the DNL BsAb platform included a phase 0 clinical study of 131I-TF2 in 2 patients with suspected CRCs to characterize the clearance kinetics of the BsAb, and a first-in-patients PRID study with 111In-IMP288 in a metastatic CRC patient (94). Although optimization of the TF2 dose was necessary, highly promising dosimetry projections of PRIT with 90Y-IMP288 were reported (kidney, 1.4 cGy/37 MBq; marrow, 0.1 cGy/37 MBq) (94). Soon afterward, PRIT clinical trials with 111In/177Lu-IMP288 commenced.
SELECTED PRETARGETING ADVANCES OF THE PAST DECADE
PRIT with Biotin–Streptavidin System
In 2013, Mawad et al. reported clinical PRIT with 111In/90Y-biotin in high-risk acute myeloid leukemia/myelodysplastic syndrome patients undergoing allogenic hematopoietic cell transplantation (95). Five patients received a 0.7 mg/kg dose of anti-CD45 mAb–streptavidin followed 48 h later by a 1.3 mg/m2 dose of 90Y-biotin (median, 2.775 GBq; range, 2.294–4.144 GBq) based on 111In-biotin pretreatment dosimetry (95). No participants were withdrawn from the study because of toxicities; 2 grade 3 gastrointestinal adverse events (enterocolitis, typhlitis) were identified as unexpected and considered possibly related to PRIT, with the only grade 4 event being expected hematopoietic cell transplantation–related cytopenia (95). A single patient was in complete remission a year after protocol treatment, and the other 4 patients died of progressive disease, with a median time to relapse of 28 d (range, 12–155 d) (95).
More recently, in 2015, a trial was initiated with anti-CD20 B9E9 with 111In/90Y-biotin in patients with high-risk B-cell malignancies to evaluate the safety of combining PRIT with carmustine, etoposide, cytarabine, and melphalan chemotherapy and autologous stem cell transplantation (NCT02483000).
Paganelli et al. performed a locoregional PRIT clinical trial on 11 breast cancer patients (96). Avidin was injected around the tumor, followed by intravenous administration of 111 MBq of 90Y-biotin. Remarkably, ADA to avidin has been shown to not limit therapy (97). De Santis et al. described AvidinOX (Alfasigma S.p.A.), an avidin variant designed to prolong the tissue half-life (2 wk compared with 2 h for native avidin) and to demonstrate cellular and interstitial protein tropism for enhanced PRIT (98). In addition to showing therapeutic promise in preclinical studies (98–101), AvidinOX PRIT has been studied in clinical investigations (NCT02053324 and NCT03188328, both of which were terminated because of low recruitment).
Preclinically, PRIT development continued (e.g., with 211At-biotin-succinylated poly-l-lysine (102), and comparative efficacy of 177Lu and 90Y for anti-CD20 PRIT of B-cell lymphomas (103)). However, a head-to-head study comparing the efficacy and toxicity of anti-CD20 biotin–streptavidin PRIT and a novel BsAb PRIT platform with an ultra-high-affinity anti-yttrium-DOTA scFv antibody (C825) revealed preferred use of BsAb PRIT in future clinical trials (104,105).
BsAb Pretargeting
The DNL system has been extensively studied both preclinically (primarily in the development of additional haptens) and clinically, with multiple trials featuring DNL anti-CEA TF2 and radiolabeled IMP288. Two notable hapten developments included a novel radiolabeled/near-infrared multimodal DNL hapten, RDC018, for image-guided surgery of various carcinomas (106) and 213Bi-IMP288 for α-PRIT (107).
In 2013, a landmark phase I PRIT study was reported with 111In/177Lu-IMP288 in 20 patients with CEA-expressing CRC (108). The 177Lu-IMP288 dose was designed to deliver no more than 1.25 Gy to marrow or 15 Gy to kidneys (3.7–7.4 GBq) based on pretreatment dosimetry with 111In-IMP288. Absorbed doses were less than 1.85 cGy/37 MBq for kidney, and mean marrow dose ranged from 0.0296 to 0.222 cGy/37 MBq based on the dosing cohort (109). Tumor doses were 0.46–4.52 Gy, and red marrow doses ranged from 0.12 to 0.97 Gy (mean TI, 4.68) (110). Furthermore, they projected an approximately 25% higher marrow TI for treatment with 90Y-IMP288 instead of 177Lu-IMP288 (mean TI simulated for 90Y-IMP288, 5.41). DLT was hematologic, and no recommended phase 2 dose was indicated. Since TF2 is humanized and lacked Fc, ADAs were unexpected but observed in about 50% of the patients on repeated injection. However, a reduced infusion rate and preadministration of prophylactics were effective at reducing associated adverse events. A PRIT trial with 90Y-IMP288 in metastatic CRC patients is ongoing (NCT02300922).
Soon after the phase I PRIT study in CRC patients, a phase I PRIT study with 111In/177Lu-IMP288 on 9 patients with CEA-expressing small-cell lung cancer was reported in 2015 (111), and first-in-humans PRID with TF2/68Ga-IMP288 in MTC patients was reported in 2016 (112). In the last 2 y, additional clinical studies with TF2/68Ga-IMP288 were performed on CRC (113) and HER2-negative/CEA-positive metastatic breast cancer patients (114). In 2021, Bodet-Milin et al. reported clinical data using TF2/68Ga-IMP288 in MTC patients, demonstrating improved sensitivity for metastatic lesion detection over 18F-l-dihydroxyphenylalanine PET/CT (115). Select examples of recent clinical PRID studies with TF2/IMP288 are shown in Figure 4.
Select examples of recent clinical BsAb PRID with DNL TF2 and radiolabeled IMP288 (TF2/IMP288). (A) Scintigraphic images (axial views) of CRC patient imaged with TF2/111In-IMP288, with highly specific targeting of primary colon tumor, confirmed by CT and 18F-FDG PET/CT (B and C, respectively). (Reprinted from (108).) (D) PET image (coronal view) of MTC patient imaged with TF2/68Ga-IMP288, with maximum-intensity-projection (MIP) image showing several pathologic lesions. (Reprinted from (115).)
Alongside the DNL BsAb pretargeting system, 2 additional BsAb pretargeting approaches using ultra-high-affinity (picomolar to femtomolar) antichelate mAbs have made significant progress in the last decade. Orcutt et al. affinity-matured the anti-DOTA chelate mAb 2D12.5 (62) and reformatted it as an scFv called C825 (116). C825 was shown to bind DOTA complexes of lutetium, yttrium, and gadolinium with similar affinity (low-picomolar range) (116). Also, Orcutt reported a highly modular tetravalent IgG-scFv BsAb format (117) and carefully screened candidate DOTA-radiohaptens to determine which had the most favorable in vivo properties for PRIT, reporting estimated human liver, kidney, and red marrow doses for 90Y-labeled haptens of 0.130–0.192, 0.703–0.999, and 0.029–0.048 cGy/37 MBq respectively (118). Also, in combination with a dextran-hapten CA, highly efficient anti-CEA mAb/C825 pretargeting with 177Lu- or 111In-haptens in nude mice bearing LS174T human CRC xenografts was demonstrated (119). These studies led to the antichelate BsAb platform called DOTA-based PRIT, or DOTA-PRIT. Between 2014 and 2018, Cheal et al. demonstrated DOTA-PRIT with 177Lu-DOTA-hapten targeting a wide variety of solid tumors (antigen targets: GD2 (120), glycoprotein A33 (121), HER2 (122)). More recently, that team developed a 225Ac-DOTA-hapten for α-PRIT (123). When DOTA-PRIT was used to pretarget 177Lu-DOTA-hapten in nude mice bearing human xenografts, TIs of 28–142 for blood, 7–23 for kidney, and 12–47 for liver were achieved, with estimated tumor-absorbed doses ranging from 1,476 to 8,473 cGy/37 MBq (120,122,124). Notably, the lowest TIs (blood, 28; kidney, 7; and liver, 12) and estimated tumor dose (1,476 cGy/37 MBq) were observed for the anti-HER2 DOTA-PRIT system, which is known to internalize (122). Green et al. also used C825 BsAb for pretargeting 90Y-DOTA-hapten to a variety of hematologic cancer targets (e.g., CD20 (104), CD45 (125), and CD38 (126)), reporting TIs of 6.75–21.4 for blood, 15.9–24.9 for kidney, and 5.52–7.24 for liver, with estimated tumor-absorbed dose ranging from 3,981 to 7,781 cGy/37 MBq.
Santich et al. reported a novel BsAb platform designed as a fusion of a self-assembling-and-disassembling (SADA) domain to a tandem single-chain BsAb for highly efficient 2-step radiohapten pretargeting (Fig. 3B) (127). With anti-GD2 SADA-PRIT plus 177Lu-DOTA-hapten in nude mice bearing human cancer xenografts, an exceptional balance of high tumor targeting and high TIs was achieved: TIs were 100 for blood, 25 for kidney, and 32 for liver, with estimated tumor-absorbed dose of 11,840 cGy/37 MBq. Also, safe and tumoricidal anti-GD2 SADA-PRIT plus 177Lu-DOTA-hapten or 225Ac-DOTA-hapten was established (127). Initial clinical trials of anti-GD2 SADA-PRIT plus 177Lu-DOTA-hapten are planned for this year on patients with recurrent or refractory metastatic GD2-expressing solid tumors, including small-cell lung cancer, sarcoma, and malignant melanoma (NCT05130255).
In 2018–2019, the team of Hoffmann-La Roche, Inc., and Orano Med LLC described a novel BsAb antitumor/antichelate hapten pretargeting system (antigen targets: CD20, HER2, and CEA) based on an anti-1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane (DOTAM) antibody with femtomolar affinity for lead-DOTAM complexes (128). Specifically, for the anti-CEA/DOTAM BsAb PRIT-0213, they reported dissociation constants of 0.84 pM and 5.7 pM for lead-DOTAM and bismuth-DOTAM, respectively (128). In nude mice bearing human cancer xenografts, they reported dosimetry for 3-step pretargeting (i.e., with CA) of 0.74 MBq of 212Pb-DOTAM. On the basis of relative biologic effectiveness equal to 5, they estimated an absorbed dose of 99.55 Gy to BxPC3 tumor and TIs of 28, 14, and 91 for blood, kidney, and liver, respectively (128). Also, they highlighted preclinical anti-CEA PRIT therapy results, detailing strong tumor growth inhibition and significantly prolonged survival with 3 cycles of 1.11 MBq (129).
Harnessing Affibody (Affibody AB) Molecules as PRIT Vectors
Although IgG-based mAbs and BsAb have traditionally been used as PRIT vectors, their prolonged circulation can make timing of complete clearance difficult, leading to unintended bystander toxicity. Advances in protein engineering have given rise to alternative protein constructs such as minibodies (80 kDa), diabodies (50 kDa), and engineered scaffold proteins (4–20 kDa) (6). These smaller constructs have proven to be especially favorable as radionuclide imaging vectors (6). Affibody molecules are a highly promising class of engineered scaffold proteins that can be optimized to have high affinity and slow internalization kinetics. The anti-HER2 Affibody radiolabeled with 111In (111In-ABY-025) has been investigated clinically for imaging of disseminated HER2-expressing breast cancer (130). However, as vectors for radioimmunotherapy, Affibody molecules have been shown to be suboptimal for residualizing radiometal labels, leading to significant renal uptake (131).
Between 2016 and 2021, numerous studies describing Affibody PRID and PRIT were reported (132,133). Figure 5 illustrates and contrasts the pretargeting systems: first is an approach using synthetic DNA-analog peptide nucleic acids (PNAs) originally described by Hnatowich et al. in 1997 for PRID with 99mTc (35), and further work applying a bioorthogonal approach using click chemistry (described in the following section). A PNA strand is covalently conjugated to a targeting vector, in this case, an Affibody, and the radionuclide payload is delivered on a complementary PNA strand, injected after an optimized pretargeting interval of 16 h (132). The hybridization of complementary PNAs as a pretargeting technique supplies several advantages, including high affinity (picomolar dissociation constant), low immunogenicity, and resistance to in vivo degradation (132). For the Affibody-PNA approach, they reported for PRIT with 177Lu-PNA (177Lu-HP2) in nude mice bearing human cancer xenografts an estimated absorbed dose of 3,996 cGy/37 MBq to tumor and TIs of 269, 5, and 81 for blood, kidney, and liver, respectively (132). These results were achieved using a 2-step approach, that is, without using CA. PRIT treatment efficacy was established using a fractionated PRIT approach (total administered 177Lu-HP2, 96 MBq/mouse) in nude mice bearing subcutaneous SKOV-3 xenografts. Median survival was significantly prolonged in PRIT-treated mice in comparison to controls, and treatment was well tolerated, with no nephrotoxicity (132). Interestingly, they reported less efficient Affibody-PRIT using the bioorthogonal chemistry approach; although mouse dosimetry was not reported, the tumor-to-kidney AUC ratio was only 1.4 (133). However, this was a marked improvement from conventional Affibody-radioimmunotherapy, which results in a tumor-to-kidney AUC ratio of less than 1 and shows the promise of PRIT to substantially reduce radiometal accumulation in the kidneys with engineered scaffold proteins (132).
Bioorthogonal Pretargeting
Meares’ elegant affinity capture pretargeting strategy, consisting of an antibody/ligand pair with complementary reactive groups that become covalently linked when they are near the antibody/ligand complex, was applied to antichelate mAb-based reporter gene imaging for noninvasive tracking of chimeric antigen receptor T cells by Krebs et al. (134). In contrast to affinity capture, with the very rapid kinetics of the bioorthogonal inverse electron-demand Diels–Alder click reaction (k2 > 103 M−1s−1), probe attachment can occur by direct reaction with a chemically modified antibody (135,136). In 2010, initial in vivo PRID studies with the inverse electron-demand Diels–Alder reaction were reported (137). Soon after, many groups took an interest. Early development efforts included novel tetrazine-based radioprecursors for numerous radioisotopes, second-generation radiolabeled tetrazine molecules with improved pharmacokinetic and biodistribution properties, and incorporation of a chase step or CA (reviewed recently by Rondon and Degoul (138)).
Significant progress has also been made for PRIT using transcyclooctene-modified mAbs and radiolabeled tetrazine for inverse electron-demand Diels–Alder. In 2017, PRIT was demonstrated using 212Pb-tetrazine in mice bearing TAG-72–expressing human CRC xenografts (139). Between 2017 and 2018, PRIT using 177Lu-tetrazine (140,141) and 225Ac-tetrazine (142), respectively, was demonstrated in multiple human tumor xenografts (CRC and pancreatic). For PRIT with 177Lu-tetrazine in nude mice bearing human xenografts, TIs of blood (10.4–17.7), kidney (12.0–19.9), and liver (19.0–40.1) were achieved, with estimated tumor-absorbed doses ranging from 9,472 to 30,821 cGy/37 MBq (140,143). Moreover, PRIT with the highly promising 64Cu/67Cu pair was recently reported (144), with the authors nicely demonstrating efficient sequential radiolabeled tetrazine administration and safe and effective theranostic treatment. For PRIT with 67Cu-tetrazine, the authors reported TIs of 6.5–9.7 for blood, 7.2–9.1 for kidney, and 4.0–4.2 for liver, with estimated tumor-absorbed doses ranging from 2,116 to 2,331 cGy/37 MBq depending on the pretargeting interval (144).
Recent efforts at bioorthogonal pretargeting development have focused on a combination of new chemistries (e.g., adamantane/cucurbituril (145)), new vectors (e.g., with nanoparticles (146); also, more details are provided in a recent review (147)), and continued optimization of radiolabeled tetrazine (148). Clinical bioorthogonal pretargeting chemotherapy trials are ongoing (NCT04106492), and bioorthogonal PRID trials are planned for the near future (149).
A ROAD MAP FOR DEVELOPMENT: RADIOBIOLOGIC GOALS OF PRIT
Our goal for PRIT should be to cure the tumor while safeguarding against excessive toxicity to normal tissues. To achieve this, we need better methods for dosimetry of internal radioemitters. We suggest that Primer 2020, the Medical Internal Radiation Dosimetry Committee publication soon to be released by the Society of Nuclear Medicine and Molecular Imaging, has provided us with the computational basis for tumor and normal-tissue dosimetry measurement to meet the goal of safe and efficacious targeted radiotherapy of advanced human tumors. Supplemental Figure 1 and the other supplemental information provide a more detailed rationale (supplemental materials are available at http://jnm.snmjournals.org).
As a starting point for radiation doses needed for curing while avoiding catastrophic damage to normal tissue, we propose the following. In a prior publication, we suggested quantitative radiobiologic targets to be met to achieve a high probability of cure for solid human tumors (1). The reasoning is that a sufficient radiation dose (cGy) must be absorbed by all cells in the tumor while minimizing the dose absorbed by normal tissues (i.e., the maximizing of TI). A series of reasonable benchmarks for effective treatment can be provided on the basis of laboratory and clinical experience, even though the response to radioimmunotherapy varies depending on several factors, including tumor size, target density, tumor structure (e.g., liquid or solid, tumor microenvironment), the type of radiation administered, the heterogeneity of targeting at the microscopic level, and the ability to repair radiation damage.
A curative watershed for PRIT of solid tumors is the set of benchmarks that achieves a cumulative total of 8,000–10,000 cGy of absorbed radiation dose to individual tumor lesions while at the same time minimizing the dose to radiosensitive tissues, such as bone marrow (<150 cGy; TI, 40–100), small intestine (<250 cGy; TI, 40–60), and kidney (<1,500 cGy; TI, 6–10) (1). Two critical parameters to control for are tumor size and target density, because these directly influence tumor dose and TIs. We have called this collective of PRIT achievable properties the sweet spot. As shown in Tables 1 and 2, various forms of PRIT have the chance to achieve the sweet-spot high tumor radiation dose and high TIs. Real-life examples from living systems support these ideas. These include the effectiveness of 131I-NaI, a medium-energy β-emitter in certain thyroid cancers (150), and the cures achieved without histopathologic evidence of radiotoxicity in animal tumor models of human xenografts.
CONCLUSIONS AND OUTLOOK
PRIT is a form of targeted radiotherapy with internally administered radioemitters (radionuclide endotherapy, or unsealed radionuclides). As we have used the term here, an antitumor vector (e.g., Affibody, mAb, or nanoparticle) is the basis for tumor targeting and is modified to achieve desired properties of radionuclide capture during the targeting procedure. PRIT is a groundbreaking achievement because its components can be delivered in a time sequence that maximizes radiation to tumor while minimizing radiation to normal tissues. In this review, we have illustrated the current status of PRIT, with an emphasis on radiohapten capture (e.g., reversible binding using antihapten BsAb or biotin–streptavidin binding), bioorthogonal techniques (irreversible binding), and Affibody-PNA pretargeting that have shown high TIs in animal tumor models of human xenografts. In all 3 approaches, modified antitumor proteins provide the targeting specificity to tumor. As shown in Figure 1, other antitumor vectors and radionuclide forms for complementary radioligand capture, are proliferating.
During PRIT, the targeting vector and the radiohapten/carrier radiopharmacology are key parameters for high TI. Building on advances in protein engineering and mAb humanization, previous obstacles such as insufficient mAb/hapten affinity and immunogenicity can be overcome. Furthermore, we can more precisely balance the attributes of affinity, molecular size, and physicochemical properties of antibody-based carriers to improve tumor localization and penetration while limiting retention in normal tissues via rapid renal clearance. Also, with innovation in BsAb design, sufficient contrast and TI can be achieved without the aid of chase or CA, which would greatly simplify dosing to 2 steps and reagents.
Another exciting new frontier is high–linear-energy-transfer radiation, in which fewer quantitative guideposts for PRIT exist. There is an unmet need to define the dosimetry more completely, with α-emitters such as 225Ac showing great promise (151). Work to estimate relative biologic effectiveness during PRIT with α-emitting radioisotopes is beginning (152).
In summary, pretargeting in nuclear medicine has achieved many milestones (Supplemental Table 1), including early-phase clinical testing (Supplemental Table 2). Pioneering clinical PRIT studies have demonstrated safety and that meaningful tumor doses can be achieved in select patients, but dosing is typically limited by insufficient TI, ADA, and the complexity of the approach. Still, clinical investigation of PRIT and radiotheranostics demonstrates increasing value for patient selection and treatment planning, permitting optimized reagent dosing during PRIT (Table 1). It is likely that individualized dosimetry will predict an optimized dose that will reduce the risk of underdosing tumors (leading to treatment failure) and overdosing normal tissues (leading to radiation toxicity).
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.
- 8.
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.
- 100.
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.
- 157.
- 158.
- Received for publication March 28, 2022.
- Revision received June 7, 2022.