Abstract
The expression status of human epidermal growth factor receptor type 2 (HER2) predicts the response of HER2-targeted therapy in breast cancer. ABY-025 is a small reengineered Affibody molecule targeting a unique epitope of the HER2 receptor, not occupied by current therapeutic agents. This study evaluated the distribution, safety, dosimetry, and efficacy of 111In-ABY-025 for determining the HER2 status in metastatic breast cancer. Methods: Seven patients with metastatic breast cancer and HER2-positive (n = 5) or -negative (n = 2) primary tumors received an intravenous injection of approximately 100 μg (∼140 MBq) of 111In-ABY-025. Planar γ-camera imaging was performed after 30 min, followed by SPECT/CT after 4, 24, and 48 h. Blood levels of radioactivity, antibodies, shed serum HER2, and toxicity markers were evaluated. Lesional HER2 status was verified by biopsies. The metastases were located by 18F-FDG PET/CT 5 d before 111In-ABY-025 imaging. Results: Injection of 111In-ABY-025 yielded a mean effective dose of 0.15 mSv/MBq and was safe, well tolerated, and without drug-related adverse events. Fast blood clearance allowed high-contrast HER2 images within 4–24 h. No anti–ABY-025 antibodies were observed. When metastatic uptake at 24 h was normalized to uptake at 4 h, the ratio increased in HER2-positive metastases and decreased in negative ones (P < 0.05), with no overlap and confirmation by biopsies. In 1 patient, with HER2-positive primary tumor, 111In-ABY-025 imaging correctly suggested a HER2-negative status of the metastases. The highest normal-tissue uptake was in the kidneys, followed by the liver and spleen. Conclusion: 111In-ABY-025 appears safe for use in humans and is a promising noninvasive tool for discriminating HER2 status in metastatic breast cancer, regardless of ongoing HER2-targeted antibody treatment.
Today, treatment of breast cancer is based on the biologic profile of the individual tumor. Knowledge of the human epidermal growth factor receptor type 2 (HER2) status is crucial to predict the response of HER2-targeted therapy (1). Patients with breast cancer overexpressing HER2 have improved survival when treated with HER2-targeting agents such as trastuzumab, pertuzumab, and trastuzumab emtansine (2–10).
The analysis of HER2 expression is usually based on a surgical specimen of the primary tumor or, in case of neoadjuvant therapy or inoperable disease, on a biopsy sample from the tumor (11). The pathologic analysis includes immunohistochemistry and in some cases fluorescence in situ hybridization (FISH). Therapy for patients with disseminated disease is often based on histopathologic classification of the primary tumor and not of the metastases. Disparities in HER2 expression of primary breast cancer and metastases have been reported. Metaanalysis of 26 studies including 2,520 patients revealed discordance in HER2 expression between the primary tumor and local lymph node metastases in the range of 2.4%–7.2% and discordance with distant metastases in the range of 6.9%–18.6%, with an absolute variation for all studies in the range of 0%–40% (12). A recent symposium publication including 2,845 patients reported absolute variations in the same range (13). Another recent study on 182 patients, with 28% discordance, indicated that patients with loss of HER2 expression in metastases had shorter overall survival than patients with unchanged expression (14).
The biopsy procedure can be inconvenient or even harmful for the patient, demanding lesions of suitable size and carrying the risk of sampling errors. Heterogeneity of HER2 expression within lesions and differences in expression between lesions in the same patient further limit the use of biopsy for correct diagnosis. Thus, improved methods for determining the HER2 status in patients with metastatic breast cancer are needed to optimize treatment regimes. One approach is molecular imaging using a radiolabeled tracer targeting HER2.
Molecular imaging allows whole-body detection of aberrant gene expression (i.e., proteomic abnormalities). Radiolabeled trastuzumab has been clinically evaluated as a HER2-specific molecular imaging agent (15,16). We used an imaging molecule with about 23 times smaller molecular weight, that is, Affibody molecules (Affibody AB), and preclinical studies have shown promising results (17). Affibody molecules are small, approximately 6.5-kDa, imaging agents based on a nonimmunoglobulin scaffold. Target-specific Affibody molecules are selected from a library of several billion unique variants providing high-affinity binders to a variety of targets such as HER2 and have shown good imaging properties in xenograft models (17–23). The HER2-binding Affibody molecule used in this study binds with picomolar affinity to the extracellular domain 3 of the receptor, that is, to an epitope not overlapping with the epitopes for trastuzumab (domain 4) or pertuzumab (domain 2), thus permitting imaging during ongoing antibody therapy (20,24,25).
Recently, clinical data using the first-generation HER2-binding Affibody molecule, ABY-002, demonstrated the feasibility of HER2 imaging with SPECT (111In) and PET (68Ga) in breast cancer patients (26). However, high liver uptake prevented visualization of liver metastases. ABY-025, used in the present clinical study, is a second-generation Affibody molecule with improved biochemical and biophysical characteristics, designed by protein engineering using an iterative approach of changing 11 amino acids (about 20% of the molecule) outside the HER2-binding region (18,27).
In this first-in-human study with 111In-ABY-025 SPECT/CT, we evaluated safety and tolerability and explored uptake in tumor metastases and background uptake in normal organs. It was also of interest to study the ability of 111In-ABY-025 to discriminate between HER2-positive and -negative metastases.
MATERIALS AND METHODS
Patients
Seven female patients (mean age, 61.3 y; range, 46–70 y) receiving treatment for recurrent metastatic breast cancer were enrolled into the study (Table 1). Five of the patients were diagnosed with HER2-positive primary tumors, and 2 had HER2-negative tumors and served as controls.
Patient Characteristics Before Injection with 111In-ABY-025
Inclusion and Exclusion Criteria
The protocol criteria for inclusion and exclusion are detailed in supplemental material (available at http://jnm.snmjournals.org). Briefly, patients with a diagnosis of metastatic breast cancer and a known HER2 classification of the primary tumor (HER2-positive: score of 3+ using HercepTest [DAKO] or FISH-positive, or score of 2+ with HercepTest and FISH-positive; HER2-negative: score 0 or 1+ using HercepTest, or score of 2+ but FISH-negative) were potential participants. Ongoing treatment was not an exclusion criterion.
Approvals
The Swedish Medical Products Agency, the regional ethics committee in Uppsala, and the radiation protection ethics committee in Uppsala approved the study. Written informed consent was obtained from all participants. The study was registered as a clinical trial with the identifiers EudraCT 210-021078-12 and NCT01216033.
Patient Characterization and Safety Assessment
In accordance with the study protocol, all patients underwent physical examination at least 7 d before, immediately before, and 7 d after injection of 111In-ABY-025. The standard clinical chemistry of blood and urine was investigated according to the approved protocol. Possible adverse effects were investigated orally and with written patient questionnaires before and after 111In-ABY-025 injection (day 0) and 1, 7, 21, and 42 d later. Blood samples for determination of shed serum HER2 were taken immediately before injection, and the assays (ADIVA Centaur HER2/neu test; Siemens Healthcare Diagnostics) were thereafter performed at Laboratory Limbach, Heidelberg, Germany. The presence of anti-ABY-025 antibodies was determined in samples taken before, 21 d after, and 42 d after injections, using an ELISA developed by Affibody AB and performed at Clinical Chemistry and Pharmacology Laboratory at Uppsala University Hospital, Sweden.
18F-FDG PET/CT and Other Clinical Imaging
The metastatic status of patients was known before inclusion on the basis of conventional imaging. 18F-FDG PET/CT imaging was performed 5 d before the 111In-ABY-025 administrations to identify the size and location of viable metastases in all patients. The patients fasted 6 h before 18F-FDG injection. A scan (Discovery VCT; GE Healthcare) from head to thighs was performed 3 h after intravenous injection of 5 MBq of 18F-FDG. A low-dose CT scan (auto-mA, 20–80) without contrast enhancement was used for attenuation correction and anatomic localization. PET images were reconstructed using a clinical protocol supplied by the vendor, and all relevant corrections for quantitative imaging were applied. The acquired data were evaluated using Hermes Hybrid Viewer (Hermes Medical) and an Advance workstation (GE Healthcare). For each lesion detected by PET/CT, the maximum standardized uptake value was noted. The volume of each tumor lesion was calculated by a thresholding technique that included all voxels with at least 42% of the maximum standardized uptake value. Additionally, MR, ultrasound, or contrast-enhanced CT was applied when needed for biopsies and further patient management.
111In-ABY-025 Imaging
111In-ABY-025 was prepared essentially as described earlier (18). ABY-025 of good-manufacturing-practice grade was provided by Affibody AB in vials containing 100 μg. ABY-025 was labeled with 111In at the Department of Nuclear Medicine, Uppsala University Hospital. Patients were not required to fast before injection. 111In-ABY-025, about 100 μg, with a mean activity of 142.6 MBq (range, 131–154 MBq), was injected intravenously. At 4, 24, and 48 h after injection, whole-body planar scanning was performed followed by SPECT/CT (Infinia Hawkeye 4; GE Healthcare) over individually selected areas of special interest, defined by findings in an initial planar scan (anterior and posterior) after about 30 min, as well as 18F-FDG PET/CT. Low-dose CT scans without contrast enhancement were acquired for attenuation correction and anatomic correlation. SPECT data were reconstructed with CT-based attenuation correction into a 128 × 128 matrix using an iterative reconstruction algorithm.
Blood Samples and Biopsies
Blood samples were collected at 10 and 30 min; at 2, 6, 24, and 48 h; and at 7 d after injection to determine blood clearance kinetics. After the SPECT/CT results were known, optional biopsies were taken from suitable and clinically relevant lesions. In 2 patients (patients 1 and 2), metastases were surgically removed after the study. Biopsies were analyzed by immunohistochemistry (HercepTest) to verify the HER2 status.
Statistical Analysis
Quotients between the 24/4- or 48/4-h uptake of 111In-ABY-025 were calculated, and 108 metastatic lesions larger than 1.5 mL, as measured by 18F-FDG PET/CT, were included in further analysis. The significance of differences between 4- and 24-h uptake values in HER2-positive metastases was analyzed using nonparametric Kruskal–Wallis 1-way ANOVA. The significance of differences between quotient values and maximum standardized uptake value for HER2-positive and HER2-negative metastases was analyzed using the nonparametric Mann–Whitney U test. A 2-sided P value of less than 0.05 was considered significant.
Supplemental Material
Information on protocol inclusion and exclusion criteria, patient medical history, blood kinetics determination, 111In-ABY-025 imaging of normal-tissue uptake, dosimetry, the dual-time-point analysis, metastatic maximum standardized uptake value measurements, and biopsy data (including immunohistochemistry and evaluation criteria) are provided in the supplemental material.
RESULTS
Safety Assessment
The administration of 111In-ABY-025 was well tolerated. No clinically significant changes in laboratory evaluations or vital signs were recorded. No anti-ABY-025–specific antibodies could be detected in any of the patients 3 and 6 wk after exposure.
Pharmacokinetics, Biodistribution, and Dosimetry
The blood kinetics of 111In-ABY-025 and uptake in the kidney, liver, and spleen are presented in Tables 2 and 3 and in Supplemental Table 1. Clearance of 111In from the blood was biphasic, with the first half-life being 2.9 ± 0.5 h and the second half-life 27 ± 5 h. In normal organs, the highest uptake was observed in the kidney, followed by the liver and spleen. Uptake in the salivary glands and bowels was also visualized. No correlation was found between any organ uptake and shed serum HER2 (8.8–56 μg/L; the upper limit of normal is 15 μg/L, Table 1). The normal organ receiving the highest radiation dose was the liver, followed by the kidneys and spleen, at 0.068 ± 0.025, 0.020 ± 0.006, and 0.005 ± 0.002 mSv/MBq, respectively. The effective radiation dose for the patients was 0.15 ± 0.02 mSv/MBq (21 mSv per patient).
Uptake of 111In in Tumor-Free Areas of Organs with Highest Uptake on SPECT Images After Injection of 111In-ABY-025
Level of 111In in Blood Samples After Injection of 111In-ABY-025
Uptake in Metastases
In the HER2-positive patients, large metastases (>1 cm) identified by 18F-FDG PET/CT could be visualized with 111In-ABY-025 in the first whole-body planar scan approximately 30 min after injection. Most metastases could be detected with high quality on images taken 4, 24, or 48 h after injection (Table 4). The mean uptake of 111In-ABY-025 in HER2-positive metastases was at least 1 order of magnitude higher than a calculated uptake for homogeneous distribution of radioactivity in the body at 24 h after injection. The HER2-positive metastases were visualized in different locations and tissues (examples for patients 1, 2, 3, and 7 are shown in Fig. 1). A brain metastasis of patient 2, not seen with 18F-FDG PET, was clearly visualized with 111In-ABY-025 and was confirmed as HER2-positive by immunohistochemistry after surgical removal. The high HER2 expression in an adrenal gland metastasis in patient 3 was detected despite its proximity to the kidney. Bone metastases were clearly visualized in patients 2, 3, and 7. Patient 7 allowed biopsy of 1 bone metastasis, and HER2 positivity was confirmed by immunohistochemistry. Liver metastases were visualized in patient 2 (Supplemental Fig. 1). Biopsies from all 4 patients (1–3 and 7) taken from lesions defined with 111In-ABY-025 SPECT were HER2-stained and scored HER2 3+.
Metastases Analyzed for 111In-ABY-025 Uptake Using SPECT/CT at Different Times After Injection
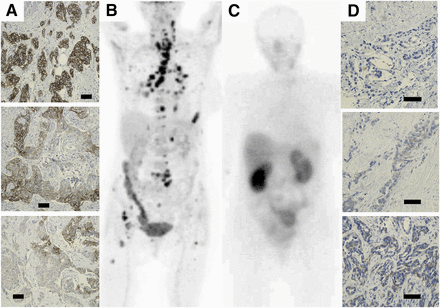
Examples of imaging of HER2 expression in breast cancer metastases using 111In-ABY-025 SPECT/CT. Rows A, B, C, D, and E correspond to patients 1, 2, 3, 4, and 7, respectively. Left column shows positive immunohistochemistry (IHC) staining of primary tumors, second column 18F-FDG PET/CT scans, third column 111In-ABY-025-SPECT/CT scans, and fourth column immunohistochemistry staining of metastases. Blue circles indicate sites where biopsies were taken: patient 1, lymph node metastasis; patient 2, brain metastasis; patient 3, adrenal metastasis; patient 4, bone metastasis; and patient 7, bone metastases. Primary tumor from patient 4 showed immunohistochemistry 3+ staining, and bone metastasis in spinal process showed high uptake of 18F-FDG. However, there was low (nearly no) uptake of 111In-ABY-025 in metastasis, indicating HER2 negativity, and this was confirmed by immunohistochemistry-negative staining of biopsy sample. Patients 5 and 6 were HER2-negative in immunohistochemistry analysis of primary tumor, in 111In-ABY-025 scans of metastases, and in immunohistochemistry of metastases (immunohistochemistry only from patient 5; patient 6 refused biopsy). Bars in all immunohistochemistry images correspond to 50 μm.
Patient 4 was included as a HER2-positive patient on the basis of an immunohistochemistry score of 3+ for the primary tumor. However, 111In-ABY-025 SPECT showed low or no uptake in the 18F-FDG–defined lesions, and the HER2-negative status of biopsies from these lesions was confirmed by immunohistochemistry (Fig. 2 and supplemental material). Patients 5 and 6 had HER2-negative primary tumors; uptake of 111In-ABY-025 in their metastases was of low contrast, and the HER2-negative status was verified by immunohistochemistry on a biopsy sample from patient 5 (patient 6 refused biopsy).
Patient 4 changed from HER2-positive primary tumor to HER2-negative metastases. Column A shows examples of variations in HER2 expression (immunohistochemistry) in primary tumor. More than 10% of tumor cells in primary tumor had strong circumferential HER2 staining of their entire cell membrane, and patient was therefore declared HER2-positive. B shows 18F-FDG PET/CT scan 5 d before SPECT. In total, 71 metastases were detected (Table 4). C shows HER2 scan 4 h after injection of 111In-ABY-025. No HER2-expressing metastases could be detected in this scan, but 3 metastases with low HER2 expression could be detected in SPECT/CT sections after 4 h and more were detected after 24 h (Table 4). However 24/4-h quotients indicated that all these metastases were HER2-negative, as verified by immunohistochemistry analysis of biopsy samples. Column D shows examples of immunohistochemistry analyses of 3 different metastases: thyroid metastasis that scored 0 (top), calcified thyroid metastasis that scored 0 (middle), and bone metastasis that scored 1+ (bottom). Bars in immunohistochemistry images correspond to 50 μm.
Discrimination Between Metastases with High and Low HER2 Expression
The quantitative 111In-ABY-025 uptake in metastases classified as HER2-positive (patients 1, 2, 3, and 7) and HER2-negative (patients 4, 5, and 6) was different at both patient level and lesion level. The uptake in HER2-positive metastases increased between 4 and 24 h, whereas the uptake in negative metastases generally was lower and decreased between 4 and 24 h. HER2-positive and HER2-negative lesions could be discriminated by calculating the decay-corrected 24/4-h uptake quotient. HER2-positive and HER2-negative metastases invariably showed a quotient greater than 1 and less than 1, respectively (Fig. 3). The difference between the groups classified as HER2-positive and HER2-negative was significant using a rank test (P < 0.05), with no overlap between the groups. The discriminatory capacity of this quotient was verified by immunohistochemistry on biopsied lesions.
Plots of 111In-ABY-025 uptake ratios in metastatic lesions defined by 18F-FDG PET/CT. In total, 108 lesions were included in analysis. (A) Average 111In uptake at 24 and 48 h calculated for each patient with HER2-positive metastases and normalized to uptake at 4 h after injection. 111In uptake increased significantly in all lesions from 4 h (●) to 24 h (■) and remained increased at 48 h (▲) after injection (Kruskal–Wallis ANOVA, P = 0.01). (B) Average 111In uptake at 24 h normalized to uptake at 4 h for patients 1, 2, 3, and 7 with HER2-positive metastases (■) and for patients 4, 5, and 6 with HER2-negative metastases (□). There was no overlap between values for HER2-positive and HER2-negative metastases, and difference was significant (Mann–Whitney, P < 0.05). Mean values and SE estimates are given. Arrow in B indicates data for patient 4, that is, patient who had HER2-positive primary tumor but HER2-negative metastases.
Reevaluation of the original primary tumor tissue from patient 4 showed a heterogeneous HER2 expression, varying from 0 to 3+ (immunohistochemistry examples are shown in Figures 2A, 2B, and 2C), but the tumor was scored 3+ since more than 10% of the cells were 3+. The analysis of 111In-ABY025 uptake in the metastases indicated low or no HER2 expression (arrow in Fig. 3), that is, were HER2-negative. Immunohistochemistry analysis of the biopsy samples from patient 4 after 111In-ABY-025 imaging showed scores from 0 to 1+ (immunohistochemistry examples are shown in Figs. 2F, 2G, and 2H). Thus, biopsy analysis of the metastases from patient 4 supported the use of 24/4-h quotients for discrimination.
DISCUSSION
The results of this first-in-human exploratory study indicate that 111In-ABY-025 can be used as a whole-body–oriented, noninvasive agent to discriminate between HER2-positive and HER2-negative metastases. A single intravenous injection was well tolerated and safe and gave an effective patient dose of approximately 21 mSv. No drug-related adverse events or anti-ABY-025 antibodies were observed.
The rapid clearance of 111In from blood and normal organs allowed HER2 imaging of large (>1 cm) metastases 30 min after injection and gave images with good contrast after 4, 24, and 48 h. The levels of shed serum-HER2 did not appear to affect normal-organ uptake or blood kinetics.
The high uptake of 111In-ABY-025 in metastases from patients 1, 2, 3, and 7 provided excellent HER2 visualization throughout the body. Immunohistochemistry analysis of biopsies confirmed the overexpression of HER2. Thus, imaging with 111In-ABY-025 can identify HER2-positive metastases. However, large lesions could also be visualized in HER2-negative patients 5 and 6, although with weak signals (Table 4). This can be explained by the fact that tumors with HercepTest scores 0 and 1+ may have up to 15,000–25,000 and 80,000–110,000 HER2 receptors per cell, respectively (28). Thus, SPECT-based imaging appears sensitive enough to visualize even low HER2 expression. Preclinical studies have shown that discrimination between tumors with high and low levels of HER2 expression is possible, either using Affibody molecules with low specific radioactivity (23) or using the fast clearance of radioactivity from tumors with low HER2 expression (29). In the present study, the decay-corrected 24/4-h uptake ratio was used to determine the HER2 status at both patient and lesion level. The average 111In-ABY-025 uptake increased significantly in all lesions from 4 to 24 h and remained increased at 48 h for HER2-positive patients 1, 2, 3, and 7. In contrast, lesions from HER2-negative patients 5 and 6 showed decreased 111In-ABY-025 uptake from 4 to 24 h. The 24/4-h uptake ratios were invariably greater than 1 for HER2-positive lesions and less than 1 for HER2-negative lesions. The validity of this approach was supported by data from patient 4 (primary tumor HercepTest score, 3+) demonstrating low 111In-ABY-025 uptake in the metastases. Analysis of all lesions from this patient showed 24/4-h quotients of less than 1, typical for low HER2 expression, and immunohistochemistry analysis of biopsies confirmed low HER2 expression with HercepTest scores of 0 or 1+.
The 24/4-h quotient method requires a 2-d protocol. A single-time-point protocol using SPECT/CT appears to be feasible (supplemental material) but may optimally require PET technique since the sensitivity and absolute quantification are better than for SPECT. Preclinical studies have demonstrated that Affibody molecules can be labeled with positron emitters such as 68Ga and 18F with preserved HER2-targeting capacity (22,30–32).
Interestingly, the use of 111In-ABY-025 allowed HER2 imaging of known liver metastases in patient 2. This is an improvement since liver metastases could not be visualized using the first-generation anti-HER2 Affibody molecule, ABY-002 (Supplemental Fig. 1) (26). ABY-025, used in the current study, has been obtained by protein engineering to increase hydrophilicity, increase thermal stability, increase production characteristics (27), and, as shown in animal experiments, lower liver uptake (18). The present study suggests that the changes engineered into ABY-025 provide clinical utility. The physiologic liver uptake varied between the patients. Patient 2 had the lowest physiologic liver uptake and fasted before administration of 111In-ABY-025, whereas patients eating before administration had higher physiologic liver uptake.
Radiolabeled trastuzumab has been evaluated earlier for HER2 imaging (15,16), and image quality has been reported to be optimal 4–5 d after injection (15), compared with 4–24 h using 111In-ABY-025. The limited number of reported clinical studies does not permit a detailed comparison of sensitivity and specificity of radiolabeled trastuzumab versus 111In-ABY-025 in the clinical setting. The unique binding epitope of ABY-025, which is different from the epitopes of either trastuzumab or pertuzumab (24), allowed imaging during trastuzumab treatment.
CONCLUSION
Our findings indicate that imaging of breast cancer metastases with 111In-ABY-025 is feasible and might be valuable for selection of patients who may, or may not, benefit from HER2-targeted therapies, hence improving treatment utility and cost-effectiveness.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. The Swedish Cancer Society provided financial support (contracts 110565 and 120415). No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank the staff of the Department of Nuclear Medicine, Uppsala University Hospital, and Research Nurse Jessica Barrefjord, Department of Oncology, Radiology, and Radiation Sciences, Uppsala University, Sweden, for administration and patient care.
Footnotes
Published online Mar. 24, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication August 22, 2013.
- Accepted for publication November 13, 2013.