Abstract
In glioma patients, differentiation between tumor progression (TP) and treatment-related changes (TRCs) remains challenging. Difficulties in classifying imaging alterations may result in a delay or an unnecessary discontinuation of treatment. PET using O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) has been shown to be a useful tool for detecting TP and TRCs. Methods: We retrospectively evaluated 127 consecutive patients with World Health Organization grade II–IV glioma who underwent 18F-FET PET imaging to distinguish between TP and TRCs. 18F-FET PET findings were verified by neuropathology (40 patients) or clinicoradiologic follow-up (87 patients). Maximum tumor-to-brain ratios (TBRmax) of 18F-FET uptake and the slope of the time–activity curves (20–50 min after injection) were determined. The diagnostic accuracy of 18F-FET PET parameters was evaluated by receiver-operating-characteristic analysis and χ2 testing. The prognostic value of 18F-FET PET was estimated using the Kaplan–Meier method. Results: TP was diagnosed in 94 patients (74%) and TRCs in 33 (26%). For differentiating TP from TRCs, receiver-operating-characteristic analysis yielded an optimal 18F-FET TBRmax cutoff of 1.95 (sensitivity, 70%; specificity, 71%; accuracy, 70%; area under the curve, 0.75 ± 0.05). The highest accuracy was achieved by a combination of TBRmax and slope (sensitivity, 86%; specificity, 67%; accuracy, 81%). However, accuracy was poorer when tumors harbored isocitrate dehydrogenase (IDH) mutations (91% in IDH-wild-type tumors, 67% in IDH-mutant tumors, P < 0.001). 18F-FET PET results correlated with overall survival (P < 0.001). Conclusion: In our neurooncology department, the diagnostic performance of 18F-FET PET was convincing but slightly inferior to that of previous reports.
Gliomas account for approximately 26% of primary central nervous system tumors and, among these, for 81% of malignant neoplasms (1). Clinical decision making considerably depends on glioma classification, based on histologic and molecular parameters (2), and imaging features. Despite some advances in surgical management and treatment regimens, grade II–IV gliomas remain incurable diseases with a decreased life expectancy.
The effectiveness of a treatment strategy is evaluated using the Response Assessment in Neuro-Oncology (RANO) criteria (3–5), which integrate MRI parameters, corticosteroid dosage, and the patient’s performance status. Nevertheless, differentiation between treatment-related changes (TRCs) and actual tumor progression (TP) continues to be a crucial issue (6). A frequent problem is the so-called pseudoprogression, which describes the phenomenon that, in the absence of actual tumor growth, the diameter of contrast-enhancing areas enlarges by more than 25% or new lesions occur during or after therapy, mimicking tumor progression within the first 3 mo after chemoradiation completion with subsequent improvement of MRI findings (7–9). Within the spectrum of TRCs, radionecrosis is also of clinical relevance. Radionecrosis denotes an irradiation-related injury to brain tissue that may occur several months or even years after radiotherapy has been completed (10,11).
Because TRCs may raise concerns about whether therapy should be initiated, continued, or changed, various imaging techniques, including MRI methods and PET, are under consideration to better distinguish TRCs from TP (12–14). In this context, PET using O-(2-18F-fluoroethyl)-l-tyrosine (18F-FET) has been shown to provide additional information (15–18) and has recently been recommended by the RANO group (19). Some studies have already investigated the performance of 18F-FET PET in glioma. However, they either were based on smaller patient populations (16,17,20–25) or included only a minor fraction of patients with TRCs (15).
In our neurooncology department, we recommended 18F-FET PET imaging when conventional MRI and clinical assessment left some ambiguity as to whether TP or sequelae of therapy prevailed. We here outline our experience and focus on the diagnostic performance of additional 18F-FET PET scans in clinical routine.
MATERIALS AND METHODS
Subjects
This retrospective study included 127 patients who were treated at the neurooncology department of the Goethe University Hospital in Frankfurt and, on the recommendation of the multidisciplinary tumor board and to distinguish between TP and TRCs, were referred to the nuclear medicine department of the University Hospital in Aachen at the Forschungszentrum Jülich for 18F-FET PET imaging between March 2016 and January 2019. The analysis was approved by the scientific board of the University Cancer Center Frankfurt and by the local ethics committee (project SNO-8-2018). All patients had undergone standard MRI before, were able to understand the reason for additional 18F-FET PET imaging, and gave written informed consent to the examination. One hundred twenty-five patients had previously been diagnosed with World Health Organization (WHO) grade II–IV glioma, and 2 patients had been treated for suspected glioma without prior biopsy.
18F-FET PET Imaging
The amino acid 18F-FET was synthetized and applied as described previously (26). All patients underwent a dynamic PET scan from 0 to 50 min after injection of 3 MBq of 18F-FET per kilogram of body weight. The interval between MRI and 18F-FET PET ranged from 0 to 77 d (median, 12 d). One hundred two patients were measured on a stand-alone PET scanner (ECAT EXACT HR+; Siemens Healthcare) in 3-dimensional mode, and 25 patients were measured on a high-resolution 3-T hybrid PET/MR scanner (BrainPET; Siemens Healthcare) (22,25). Because of the reconstruction parameters and postprocessing steps, the different scanner types did not affect the quantitative 18F-FET PET parameters (27).
Postprocessing of 18F-FET PET Images
Mean tumoral 18F-FET uptake was determined by a 2-dimensional automatic contouring process using a tumor-to-brain ratio of at least 1.6 as described previously (22,25). For maximal amino acid uptake, a circular region of interest with a diameter of 1.6 cm was centered on maximal tumor uptake (15), to exclude an influence of different scanner resolutions. Mean and maximum tumor-to-brain ratio (TBRmean and TBRmax) were calculated by dividing the SUVmean and SUVmax of the tumor region of interest by the SUVmean of a larger crescent-shaped volume of interest placed in the semioval center of the contralateral unaffected hemisphere including white and gray matter (28,29).
Furthermore, time–activity curves for 18F-FET uptake in the tumor were obtained by application of a spheric volume of interest with a diameter of 1.6 cm to the entire dynamic dataset. Time-to-peak values (minutes from the beginning of the dynamic acquisition up to the SUVmax of the lesion) were derived from time–activity curves, and the slope of the time–activity curve in the late phase of 18F-FET uptake was calculated by fitting a linear regression line to the late phase of the curve (20–50 min after injection). The slope was expressed as change in SUV per hour.
Diagnosis of Tumor Progression and TRCs
TP or TRCs were confirmed by histopathologic examination after resection or biopsy or by clinicoradiologic follow-up. For WHO grade II gliomas, both the clinical and the radiologic situation had to be stable or improved for at least 12 mo without administration of another therapy to exclude TP (16). For WHO grade III–IV gliomas, the classification of TRCs required at least 6 mo of a stable or improved clinical and radiologic condition (17), as well as no change in tumor treatment. TP was considered present when lesions continued to increase in size on at least 2 subsequent MRI scans according to the RANO criteria, paralleled by a deterioration in performance status, or when a patient died of glioma, whichever occurred first. Thus, the classification criteria in our study were similar to those of previous investigations (25,30,31) or were more stringent (20).
Neuropathology
Tissue obtained from resection or biopsy was fixed in 4% paraformaldehyde and paraffin-embedded. Sections 3 μm thick were cut on an SM 2000R microtome (Leica Biosystems), mounted on microscope slides (SuperFrost Plus; Thermo Scientific), and subjected to hematoxylin–eosin staining. Immunohistochemistry against the isocitrate dehydrogenase (IDH) mutation-specific antibody IDH1_R132H (mouse monoclonal, clone DIA-H09, concentration 1:50; Dianova) was performed according to standardized protocols using a Leica BOND-III stainer. A tumor was considered to be progressive when it was seen to be solid in histologic workup; the occurrence of single—for example, IDH1_R132H-positive—tumor cells was not sufficient for diagnosis of TP. TRCs, on the other hand, were characterized as a lack of solid tumor or the presence of radiogenic necrosis, hyalinized vessel walls, or resorptive changes.
Statistical Analysis
Data were analyzed using Excel (Microsoft), SPSS Statistics (version 25; IBM), and SigmaPlot (version 11.0; Systat Software). Continuously scaled variables were compared by the Mann–Whitney rank sum test or the Student t test for independent samples, and categoric variables were compared by the Pearson χ2 test or the Fisher exact test. Survival was calculated from the date of 18F-FET PET imaging to the date of death or the last follow-up visit, and survival distributions were analyzed using the log-rank test. Univariate and multivariate Cox regression models were applied to identify prognostic parameters. A P value of less than 0.05 was considered significant. The diagnostic performance of the 18F-FET PET parameters TBRmax, TBRmean, time-to-peak value, and slope for differentiation of TP from TRCs was assessed by receiver-operating-characteristic curve analyses using the neuropathologic results or the clinicoradiologic follow-up as a reference. The decision cutoff was considered optimal when the product of paired values for sensitivity and specificity reached its maximum. Visualization was performed using Excel, Illustrator (Adobe), and RAWGraphs (http://app.rawgraphs.io/) (32).
RESULTS
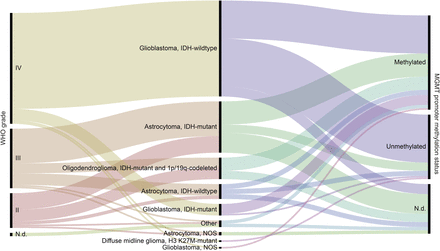
Patient and tumor characteristics are depicted in Figure 1, Tables 1 and 2, and Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
WHO grade, diagnosis according to WHO 2016 classification of brain tumors (2), and MGMT promoter methylation status of tumors that were later examined with 18F-FET PET; N.d. = not determined or inconclusive.
Patient and Tumor Characteristics, Part 1
Patient and Tumor Characteristics, Part 2
Repeated resection was performed for 36 patients and biopsy for 4 patients. The median time from 18F-FET PET to surgery was 21.5 d (range, 10–215 d) and was longer when 18F-FET PET indicated TRCs (6 patients; median, 90 d; range, 12–215 d) than when 18F-FET PET suggested TP (34 patients; median, 19 d; range, 10–119 d). Eighty-seven patients were evaluated on the basis of clinicoradiologic follow-up. Until June 2019, 57 of the 127 patients succumbed to their disease (median time from 18F-FET PET to death, 208 d; range, 24–950 d), and 70 continued follow-up (median time from 18F-FET PET to last follow-up visit, 484 d; range, 128–1,050 d).
Receiver-operating-characteristic analysis yielded a TBRmax of 1.95 as an optimal cutoff to identify TP (sensitivity, 70%; specificity, 71%; area under the curve, 0.76 ± 0.05; P < 0.001). The cutoff for the TBRmean to detect TP was also 1.95 (sensitivity, 56%; specificity, 79%; accuracy, 62%; area under the curve, 0.75 ± 0.05; P < 0.001). The time-to-peak value did not allow discrimination between TP and TRCs (area under the curve, 0.58; P = 0.15). For slope, the optimal cutoff to show TP was less than 0.2 SUV/h (sensitivity, 54%; specificity, 86%; accuracy, 63%; area under the curve, 0.69 ± 0.05; P < 0.001). The combined analysis of a TBRmax greater than 1.95 or a slope less than 0.2 SUV/h revealed TP best, with a sensitivity of 86%, a specificity of 67%, and an accuracy of 81% (P < 0.001). In individual cases (6 patients), further criteria such as a focal hotspot that was underestimated by the region-of-interest analysis, or an increasing 18F-FET uptake compared with a previous 18F-FET PET examination, were also considered indicators of TP (Supplemental Table 1). Supplemental Tables 2 and 3 summarize the diagnoses based on 18F-FET PET findings. Figure 2 depicts examples of false-positive and -negative 18F-FET PET ratings.
Examples of false-negative and -positive 18F-FET PET ratings. (A–D) A 45-y-old-patient had been diagnosed with IDH-mutant, MGMT promoter methylated glioblastoma in November 2010. After gross total resection, radiotherapy, and irinotecan chemotherapy, she received bevacizumab every other week. In January 2017, follow-up MRI indicated disease progression (RANO criteria). However, in February 2017, 18F-FET PET imaging was not suggestive of tumor, and so patient continued follow-up. Subsequent MRI revealed enlargement of both contrast-enhancing and non–contrast-enhancing lesions (tumor progression, RANO criteria), but 18F-FET PET remained negative. In November 2017, biopsy revealed tumor progression. Shown are axial MRI, October 2017, T2 image (A, left) and contrast-enhanced T1 image (A, right); 18F-FET PET, November 2017 (B); and histology (hematoxylin-eosin [HE]) (C) and immunohistochemistry (IDH1_R132H, arrowheads point to IDH1_R132H-positive tumor cells) (D) of biopsy samples, November 2017. (E–H) A 39-y-old patient had undergone subtotal resection of IDH1_R132H-mutant and 1p/19q-codeleted oligodendroglioma in August 2010, temozolomide chemotherapy until January 2011, proton therapy in May and June 2015, and lomustine chemotherapy from July to December 2015. In July 2017, putative recurrent tumor was resected. Neuropathology showed sequelae of radiation but no tumor. Shown are axial MRI, May 2017, T2 image (E, left) and contrast-enhanced T1 image (E, right); 18F-FET PET indicating tumor progression, June 2017 (F); and necrosis and calcification (arrows, HE) (G) without IDH1_R132H-positive tumor cells (H) in resected samples, July 2017. (I–K) IDH-mutant, MGMT promoter methylated glioblastoma of 38-y-old patient had been treated by partial resection in April 2016, radiotherapy, and temozolomide chemotherapy from April to June 2016. Against our advice, patient decided not to continue tumor-specific therapy. However, imaging alterations regressed spontaneously. Shown are coronal MRI, February 2017, T2 image (I, left) and contrast-enhanced T1 image (I, right); 18F-FET PET indicating tumor progression, April 2017 (J); and follow-up MRI, February 2018, T2 image (K, left) and contrast-enhanced T1 image (K, right).
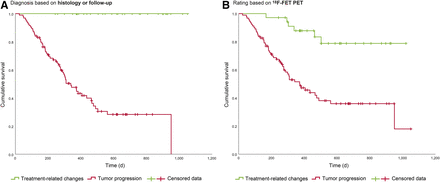
Overall survival was longer when, finally, TRCs were diagnosed (Fig. 3A), as well as when 18F-FET PET results indicated TRCs (Fig. 3B). The results of univariate and multivariate survival analyses are given in Table 3. In multivariate evaluation, we fitted a stepwise-backward exclusion model including the 18F-FET PET rating, the tumor grade, the IDH status, the O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status, the patient’s age, and the patient’s Karnofsky performance status. The 18F-FET PET rating, the WHO grade, the IDH status, and the Karnofsky performance status remained independent prognostic factors.
Overall survival of all 127 patients. (A) Overall survival after 18F-FET PET imaging, depending on whether TP or TRCs were present, as assessed by histology or follow-up (P [log-rank] < 0.001). (B) Overall survival after 18F-FET PET imaging, depending on 18F-FET PET results (P [log-rank] < 0.001).
Univariate and Multivariate Analyses of Overall Survival
Looking at the tumor characteristics, we noticed that the accuracy of 18F-FET PET was higher in IDH-wild-type gliomas than in IDH-mutant ones (P < 0.001). The diagnosis based on 18F-FET PET turned out to be incorrect in 33% of the IDH-mutant tumors (11 true-negative, 23 true-positive, 8 false-positive, and 9 false-negative results) but in only 9% of the IDH-wild-type tumors (8 true-negative, 56 true-positive, 3 false-positive, and 3 false-negative results). MGMT promoter methylation did not significantly affect the diagnostic performance of 18F-FET PET.
DISCUSSION
Diagnosis and treatment of brain tumors are strongly linked to imaging techniques, especially MRI techniques, because histologic confirmation often cannot be realized easily and without substantial risk. 18F-FET PET is not a standard method for the assessment of TP in glioma but may be more accurate than conventional MRI (14,25) and helpful in complex or challenging cases (19). In our department, we consider this method in particular when MRI yields inconclusive results. The present report outlines our experience with 18F-FET PET in differentiating TP from TRCs in WHO grade II–IV gliomas. 18F-FET PET based on TBRmax achieved an accuracy of 70%, which could be increased to 81% by combination with kinetic parameters. However, these values are in the low range compared with previous studies.
Retrospectively analyzing 132 scans of 124 WHO grade II–IV glioma patients, Galldiks et al. described an accuracy of 93% for 18F-FET PET to diagnose TP (15), but the number of patients with TRCs in that study, namely 8%, was quite small and might have influenced the results. Looking at 45 patients suspected of having TP, Rachinger et al. found a sensitivity of 100% and a specificity of 93% for 18F-FET PET imaging (21). Kebir et al. noted a sensitivity of 84%, a specificity of 86%, and an accuracy of 85% for 18F-FET PET to differentiate between TP and pseudoprogression in a series of 26 patients (20). In a study on 36 glioblastoma patients conducted by Mihovilovic et al., static 18F-FET PET discriminated between TP and TRCs with a sensitivity of 89%, a specificity of 75%, and an accuracy of 86% (31). Analyzing the 18F-FET PET scans of 48 high-grade glioma patients, Werner et al. reported a prevalence of 21% for TRCs and a 93% diagnostic accuracy for static and dynamic 18F-FET PET parameters (25). In our study, the percentage of patients with TRCs was similar to that in other studies (20,25,31), but the diagnostic performance of 18F-FET PET imaging was slightly inferior (20,23,31).
One must consider that all patients in this study were treated in a single neurooncology department with procedures that were based on weekly discussions in multidisciplinary tumor conferences. Therefore, the decision-making process should have been consistent but carried several biases. First, 18F-FET PET imaging was not part of the routine workup of patients with suspected TP. Many patients initially underwent MR perfusion and spectroscopy, and often 18F-FET PET was recommended merely in cases of ambiguity. Therefore, the patient group might represent a selection of particularly difficult cases, which in turn could lead to an underestimation of the accuracy of 18F-FET PET. Second, imaging was considered appropriate only if it resulted in therapeutic consequences. That is why patients with a poor performance status or without further treatment options were not assigned to receive 18F-FET PET imaging. Third, a higher rate of histologic confirmation after 18F-FET PET would have been desirable, but resection or biopsy was not routinely performed when the imaging aspect was ambiguous. Invasive interventions were suggested only if all evidence pointed toward TP. However, the sole inclusion of patients with histologic confirmation would lead to a different bias, especially to the exclusion of true-negative results. Despite these limitations, this study probably reflects the current situation in many centers, as 18F-FET PET is not generally available as a routine tool and can be used only in selected indications.
An interesting new observation in our study was that the accuracy of 18F-FET PET in differentiating TP from TRCs was significantly higher in IDH-wild-type tumors than in IDH-mutant ones. This knowledge could be helpful when considering 18F-FET PET as an additional diagnostic tool. Possibly, previous studies did not reveal this aspect because of a lack of molecular markers, smaller collectives, or a minor fraction of patients with TRCs. It is certainly worth further investigation and should be verified in a larger number of patients. In view of the current literature, we cannot clearly explain this finding, especially false-positive 18F-FET PET results. Compared with IDH-wild-type tumors, IDH-mutant gliomas are considered less immunologically active (33), and the presence of mutant IDH has been shown to impair complement activation, infiltration of CD45-positive immune cells, T-cell migration, proliferation, and activity (34). Because inflammation may contribute to the 18F-FET PET signal under certain circumstances (14), immunosuppression might mask tumor growth and lead to false-negative results.
CONCLUSION
18F-FET PET complemented our current diagnostic portfolio, drove decision making, and independently predicted survival. The interpretation of results should consider the tumor’s IDH status because, in our study, the accuracy of 18F-FET PET was higher in IDH-wild-type gliomas.
DISCLOSURE
The Dr. Senckenberg Institute of Neurooncology is supported by the Dr. Senckenberg foundation (grant 2014/SIN-02). Joachim Steinbach has received a grant from Merck, as well as honoraria for lectures, travel, or advisory board participation from Roche, Medac, Bristol-Myers Squibb, and Abbvie. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How well can 18F-FET PET help to distinguish between TP and TRCs?
PERTINENT FINDINGS: In this retrospective analysis of patients with WHO grade II–IV glioma treated at our neurooncology department, the diagnostic accuracy of 18F-FET PET was slightly inferior to that of previous reports and was higher in IDH-wild-type than in IDH-mutant tumors. The 18F-FET PET rating was prognostic of survival.
IMPLICATIONS FOR PATIENT CARE: 18F-FET PET provided valuable information. Our observation that its accuracy depended on the IDH status might be crucial for decision making.
Acknowledgments
We thank all the patients and their families for participating in this study; the nurses and assistants at the Dr. Senckenberg Institute of Neurooncology for supporting the outpatient procedures; Erika Wabbals, Silke Grafmueller, and Sascha Rehbein for technical assistance in radiosynthesis of 18F-FET; and Silke Frensch, Suzanne Schaden, Kornelia Frey, and Trude Plum for technical assistance in performing the PET measurements at the Forschungszentrum Jülich.
Footnotes
↵* Contributed equally to this work.
Published online Sep. 13, 2019.
- © 2020 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication August 5, 2019.
- Accepted for publication September 6, 2019.