Visual Abstract
Abstract
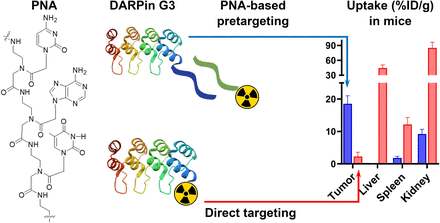
Designed ankyrin repeat proteins (DARPins) are a class of engineered scaffold proteins (ESPs) with a molecular weight of approximately 15 kDa and a picomolar affinity for tumor antigen targets. Proof-of-concept studies have demonstrated the potential of DARPin radioimmunodiagnostics in humans. However, a high accumulation of activity in the kidneys limits their use in conventional radionuclide therapy. A peptide nucleic acid (PNA)–based pretargeted approach was successfully applied to Affibody molecules, another class of ESP. We hypothesized that this method could also enable the controlled conversion of DARPins into pretargeting probes. In this proof-of-principle study, we tested this hypothesis using the human epidermal growth factor receptor type 2 (HER2)–targeting DARPin G3 as a model. Methods: We performed site-specific coupling of PNA to the DARPin using sortase A–mediated ligation. The DARPin G3 was modified at the C-terminus with a sortase A recognition sequence. A GGG–modified hybridization probe (HP1) containing a 15-base PNA sequence was attached to G3 using sortase A, creating the primary agent G3-HP1. To evaluate cell binding specificity and biodistribution, G3-HP1 was labeled with 125I. The complementary PNA-based secondary probe HP2 containing the DOTA chelator was labeled with 177Lu. In vitro studies were performed in HER2-expressing cell lines. Biodistribution and in vivo targeting and pretargeting specificity were evaluated in mice with HER2-positive SKOV-3 and HER2-negative Ramos xenografts. In vivo pretargeting of [177Lu]Lu-HP2 using G3-HP1 was head-to-head compared with Affibody molecule–mediated pretargeting using ZHER2:342-HP1 and with direct targeting using [177Lu]Lu-DOTA-G3. Results: [125I]I-G3-HP1 demonstrated specific binding to HER2-expressing cells with picomolar affinity. [177Lu]Lu-HP2 showed HER2-specific and PNA-dependent binding to G3-HP1–pretreated cells with subnanomolar affinity. Biodistribution studies confirmed HER2-specific tumor uptake of [125I]I-G3-HP1. The uptake of [177Lu]Lu-HP2 in xenografts was HER2-dependent and PNA-mediated in the case of G3-HP1 preinjection. The pretargeting approach increased the tumor uptake 8-fold compared with direct targeting using [177Lu]Lu-DOTA-G3. Pretargeting substantially decreased the uptake in the kidneys (∼9-fold), liver (∼370-fold), and spleen (6.5-fold). The biodistribution and the tumor uptake of [177Lu]Lu-HP2 were strikingly similar in the cases of Affibody- and DARPin-based pretargeting. Conclusion: Sortase A–mediated coupling enables the development of a PNA-based pretargeting system for DARPin G3, expanding the application of this approach to another class of ESPs.
Footnotes
↵* Contributed equally to this work.
Published online May 15, 2025.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
SNMMI members
Login to the site using your SNMMI member credentials
Individuals
Login as an individual user