Abstract
Hepatocyte transporters control the hepatobiliary elimination of many drugs, metabolites, and endogenous substances. Hepatocyte transporter function is altered in several pathophysiologic situations and can be modulated by certain drugs, with a potential impact for pharmacokinetics and drug-induced liver injury. The development of substrate probes with optimal properties for selective and quantitative imaging of hepatic transporters remains a challenge. 99mTc-mebrofenin has been used for decades for hepatobiliary scintigraphy, but the specific transporters controlling its liver kinetics have not been characterized until recently. These include sinusoidal influx transporters (organic anion-transporting polypeptides) responsible for hepatic uptake of 99mTc-mebrofenin, and efflux transporters (multidrug resistance–associated proteins) mediating its canalicular (liver-to-bile) and sinusoidal (liver-to-blood) excretion. Pharmacokinetic modeling enables molecular interpretation of 99mTc-mebrofenin scintigraphy data, thus offering a widely available translational method to investigate transporter-mediated drug–drug interactions in vivo. 99mTc-mebrofenin allows for phenotyping transporter function at the different poles of hepatocytes as a biomarker of liver function.
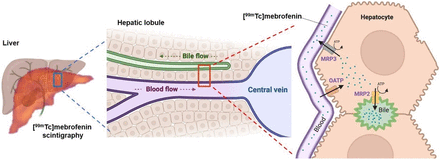
The quantitative measurement of liver function remains a holy grail of modern medicine (1). From a molecular perspective, liver function can be estimated by the activity of membrane transporters expressed in hepatocytes. An array of membrane transporters of the solute carrier (SLC) and adenosine triphosphate–binding cassette (ABC) superfamilies control the hepatobiliary clearance of many endogenous and exogenous compounds. Several SLC influx transporters, including the organic anion–transporting polypeptide (OATP) transporters OATP1B1 and OATP1B3, are expressed in the sinusoidal (blood-facing) membrane of hepatocytes, where they mediate the uptake of their substrates from blood into the liver (2). ABC efflux transporters expressed at the canalicular (bile-facing) membrane of hepatocytes, such as the multidrug resistance–associated protein 2 (MRP2) or the bile salt export pump, control the biliary excretion of solutes and bile acids (2). Other ABC transporters are expressed in the sinusoidal hepatocyte membrane, where they mediate the backflux of solutes from hepatocytes into blood (Fig. 1). Hepatocyte transporters control the hepatobiliary clearance of many drugs and metabolites. Many marketed drugs have been identified as inhibitors or substrates of hepatocyte transporters. There is an associated risk for transporter-mediated drug–drug interaction (DDI) with an impact for pharmacokinetics and drug safety (3). Consequently, regulatory authorities request that several hepatocyte transporters be investigated as potential sites for pharmacokinetic DDIs of new drug candidates (2).
Multiscale physiologic and molecular interpretation of liver kinetics of 99mTc-mebrofenin. ATP = adenosine triphosphate.
Imaging using substrate probes for hepatic transporters bears great potential to provide molecular insight into liver function (4). However, the development of imaging probes for selective and quantitative imaging of hepatic transporters is a challenge, as described in recent reviews (4–6). Because candidate probes are often substrates of multiple hepatic transporters, true selectivity is difficult to achieve. Moreover, hepatic transporter substrates with predominantly liver extraction often undergo extensive metabolism, which complicates the molecular interpretation of the imaging signal measured in the liver and bile. Metabolically stable probes are therefore preferred for correct estimation of hepatic transporter function (4).
Several 99mTc-labeled radiopharmaceuticals have been approved for evaluating liver function in patients. For instance, scintigraphy with 99mTc-sulfur colloids has been used to visualize phagocytosis by the reticuloendothelial cells of the liver (7). The binding of 99mTc-labeled galactosylneoglycoalbumin to the asialoglycoprotein receptors expressed at the surface of hepatocytes is considered a biomarker of liver function (7). In addition, iminodiacetate derivatives are preferentially taken up by hepatocytes and follow intracellular paths similar to that of endogenous substances, making them suitable to assess important liver functions. 99mTc-(2,6-dimethylacetanilide)iminodiacetate was approved in the mid-1970s for hepatobiliary scintigraphy, followed by 99mTc-disofenin (99mTc-diisopropyliminodiacetic acid) and 99mTc-mebrofenin (99mTc-N-(3-bromo-2,4,6-trimethylphenylcarbamoylmethyliminodiacetic acid) (7,8). 99mTc-mebrofenin benefits from improved pharmacokinetic properties such fast blood clearance caused by rapid and predominant uptake by the liver followed by biliary excretion, with minimal urinary excretion (9,10). Radiolabeling of 99mTc-mebrofenin from commercial kits is straightforward, yielding high radiochemical purity and stability (11). 99mTc-mebrofenin scintigraphy is therefore widely used in nuclear medicine to explore the dynamics of biliary excretion or estimate hepatic extraction capacity to assess biliary disorders, such as bile duct obstruction, cholecystitis, or gallbladder dysfunction (10,12,13). 99mTc-mebrofenin is increasingly used to monitor liver function in the setting of liver surgery and transplantation, for preoperative assessment of future remnant liver function (7,14).
The factors governing liver specificity and hepatobiliary elimination of 99mTc-mebrofenin were not known when this imaging agent was initially developed (15–17). The characterization of various carrier-mediated processes in hepatocytes enabled identifying the transporters involved in the liver kinetics of 99mTc-mebrofenin (5). An in-depth characterization of the hepatic transport profile of 99mTc-mebrofenin led to use of this widely available radiopharmaceutical as a probe for translational molecular imaging of hepatocyte transporters.
NONCLINICAL STUDIES ON TRANSPORT OF 99mTc-MEBROFENIN
In vitro studies on cells overexpressing selected hepatocyte transporters demonstrated that 99mTc-mebrofenin is transported by the influx transporters OATP1B1 and OATP1B3 but neither by OATP2B1 nor by Na+-taurocholate cotransporting polypeptide and organic cation transporter 1 (15,17,18). The potent OATP inhibitors rifampicin, rifamycin SV, and glycyrrhizic acid markedly decreased 99mTc-mebrofenin uptake in transporter-overexpressing cells, whereas organic cation and anion transporter inhibitors did not (17–19). Regarding efflux transporters, 99mTc-mebrofenin is transported predominantly by the ABC transporters MRP2 and MRP3 (17,19,20). Efflux transport of 99mTc-mebrofenin by MRP2 and MRP3 was inhibited by the MRP inhibitors MK571, ritonavir, and E217βG (17,19,20). The importance of carrier-mediated systems for the cellular uptake of 99mTc-mebrofenin was confirmed by studies on water-injected oocytes, in which low uptake of 99mTc-mebrofenin was observed, suggesting negligible passive diffusion across cell membranes (15).
The importance of carrier-mediated transport processes was confirmed in vivo using transporter-deficient animals. In OATP-deficient mice, more radioactivity was measured in the blood and urinary bladder, with a considerably lower signal in the liver (18). In MRP2-deficient rats and mice, biliary excretion of 99mTc-mebrofenin was reduced compared with wild-type animals, whereas liver exposure was increased despite a similar initial liver uptake (16,18). Rifampicin, which is an inhibitor of both OATP and MRP2, significantly decreased the hepatic uptake of 99mTc-mebrofenin and its transfer to the gallbladder and intestine (18). Consequently, radioactivity was higher in the blood and urinary bladder, suggesting a shift from hepatobiliary to urinary excretion. Cyclosporin A (CsA), known to inhibit several efflux transporters including MRP2, did not further decrease the biliary excretion of 99mTc-mebrofenin in MRP2-deficient rats, suggesting a limited role of other ABC transporters at the liver–bile interface (16). We recently investigated the impact of increasing doses of CsA on the liver kinetics of 99mTc-mebrofenin, revealing that CsA was more potent in inhibiting MRP2 than OATP (21). Low-dose CsA (0.01 mg/kg administered intravenously) completely inhibited the MRP2-mediated biliary excretion of 99mTc-mebrofenin without affecting the OATP-mediated sinusoidal uptake. It is noteworthy that liver exposure to 99mTc-mebrofenin was significantly increased under conditions of decreased biliary excretion, whereas no significant changes in blood radioactivity were observed. This finding illustrates that dramatic changes in the liver exposure to drugs can be observed, which may potentially lead to hepatotoxicity, with a nondetectable impact on plasma pharmacokinetics. Pharmacokinetic modeling revealed a dose-dependent inhibition of the sinusoidal efflux (liver-to-blood) of 99mTc-mebrofenin by CsA, consistent with the expression of MRP3 at this specific interface (17,21). In rats, the extent of MRP3-mediated sinusoidal and MRP2-mediated canalicular efflux transport of 99mTc-mebrofenin was similar but remained much lower than the OATP-mediated sinusoidal uptake, thus providing information regarding the relative importance of carrier-mediated processes in hepatocytes (21). Imaging probes for quantitative determination of MRP2 and MRP3 function in hepatocytes are still not available (6). The nonclinical transporter selectivity profile of 99mTc-mebrofenin suggests it may be repurposed as a translational molecular imaging probe for simultaneous exploration of OATP, MRP2, and MRP3 function at the different poles of hepatocytes (Fig. 1).
CLINICAL STUDIES WITH 99mTc-MEBROFENIN TO INVESTIGATE HEPATOCYTE TRANSPORTERS
Brouwer’s group has pioneered the use of 99mTc-mebrofenin as a phenotypic probe to explore hepatocyte transporter function in humans (17,20,22). The kinetics of 99mTc-mebrofenin in humans were first interpreted using a 2-compartment model, which suggested canalicular efflux (MRP2) rather than sinusoidal efflux (MRP3) as the rate-limiting step for controlling the plasma and liver kinetics of 99mTc-mebrofenin in humans (17). This modeling approach was also applied to simulated pathophysiologic abnormalities, such as hyperbilirubinemia and cholestasis. Mathematic simulations concluded that hyperbilirubinemia would decrease the hepatic exposure to 99mTc-mebrofenin, associated with increased blood concentrations (17), as may be explained by competition of plasma bilirubin with 99mTc-mebrofenin for OATP-mediated uptake into the liver (23). Simulations of impaired biliary excretion due to cholestasis resulted in prolonged hepatic exposure to 99mTc-mebrofenin. According to the model, cholestasis is expected to increase the blood exposure to 99mTc-mebrofenin because of extensive hepatic accumulation and increased sinusoidal efflux, suggesting involvement of MRP3-mediated sinusoidal transport (17).
This molecular imaging approach was then tested on a cohort of patients with nonalcoholic steatohepatitis (NASH) (22), in whom regulatory changes in hepatocyte transporter expression have been reported (24,25). Plasma and liver exposure to 99mTc-mebrofenin was increased in the NASH cohort by 1.4- and 1.6-fold, respectively (22). Both the hepatic uptake and the biliary efflux clearance of 99mTc-mebrofenin were reduced in NASH patients compared with healthy subjects. From a molecular perspective, this finding suggested impaired MRP2-mediated canalicular efflux transport of 99mTc-mebrofenin in NASH, consistent with previous studies finding a mislocalization of MRP2 in the canalicular hepatocyte membrane of NASH patients (25). No difference was observed in the sinusoidal efflux clearance of 99mTc-mebrofenin, although an increase in hepatic MRP3 expression and function has been previously found in NASH patients using other methods (25,26). Interestingly, liver uptake of 99mTc-mebrofenin was slightly lower in healthy subjects harboring a low- (SLCO1B1*15/*15) or intermediate-function (SLCO1B1*1A/*15) genotype of the gene encoding for OATP1B1, although liver uptake remained higher than that observed in NASH patients (22). These pioneer clinical data highlight the potential of 99mTc-mebrofenin scintigraphy with kinetic modeling to explore the functional consequences of the pathophysiologic regulation of certain hepatocyte transporters.
MOLECULAR INSIGHT INTO 99mTc-MEBROFENIN LIVER KINETICS
Knowledge of the molecular determinants of the hepatic handling of 99mTc-mebrofenin may shed light on abnormal 99mTc-mebrofenin scintigraphy results. Thirty years ago, there was a report of a patient with Rotor syndrome who exhibited decreased and delayed liver uptake of 99mTc-mebrofenin, normal biliary excretion, and persistent visualization of the cardiac blood pool, kidneys, and urinary bladder (27). The etiology of this disease is now identified and consists of mutations in the SLCO1B1 and SLCO1B3 genes, encoding nonfunctional OATP1B1 and OATP1B3 transport proteins (28). This etiology provided a mechanistic explanation for the altered liver kinetics of 99mTc-mebrofenin observed in this patient. A study on rats with inflammation-induced liver injury showed reduced hepatic uptake of 99mTc-mebrofenin as compared with control rats (29), a finding that is consistent with changes in hepatic transporter expression, including a decrease in OATP1B1 expression (30).
These data suggest that pathophysiologic modulation of hepatocyte transporter function may impact the liver kinetics of 99mTc-mebrofenin. Moreover, concomitant drug intake may also alter the hepatic disposition of 99mTc-mebrofenin. Many marketed drugs are known to inhibit hepatic transporters handling 99mTc-mebrofenin (Table 1). 99mTc-mebrofenin may therefore become an unintended victim of transporter-mediated DDIs, which may confound the interpretation of liver scintigraphy images. For instance, low hepatic clearance of 99mTc-mebrofenin was recently reported in a patient treated for hepatitis C with grazoprevir, an OATP1B1/3 substrate, suggesting competitive inhibition of hepatic uptake transport (Fig. 2) (31). CsA is a potent inhibitor of the OATP- and MRP2-mediated transport of 99mTc-mebrofenin (21). A study performed on patients undergoing liver transplantation showed that hepatic extraction of 99mTc-mebrofenin was consistently lower in CsA-treated patients than in tacrolimus-treated patients, despite similar liver function assessed using biochemistry assays (32). These reports provide evidence that concomitant drug intake may precipitate transporter-mediated DDIs with 99mTc-mebrofenin (21).
Impact of concomitant drug intake on clinical 99mTc-mebrofenin scintigraphy data. Dynamic acquisition (anterior view, 45-s summed planar images over 6 min) was acquired after injection of 99mTc-mebrofenin in 42-y-old woman treated with grazoprevir (A) and at 3 wk after end of treatment (B). Liver uptake of 99mTc-mebrofenin was markedly reduced under grazoprevir treatment, presumably because of competitive inhibition of OATP1B1/3-mediated liver uptake of 99mTc-mebrofenin. (Adapted from (31).)
On the other hand, several pharmacokinetic studies have illustrated the potential of 99mTc-mebrofenin as a probe to specifically investigate DDIs involving OATP, MRP2, and probably MRP3, at both sinusoidal and canalicular membranes of hepatocytes in drug development. The great advantage of 99mTc-mebrofenin is that it is a metabolically stable probe, which allows selective assessment of hepatocyte transporter function and is not affected by changes in the activity of metabolizing enzymes. For instance, ritonavir was shown to inhibit several hepatocyte transporters in vitro and is also a powerful inhibitor of cytochrome P450 enzymes (20). A semiphysiologically based pharmacokinetic model showed that ritonavir significantly increased systemic exposure to 99mTc-mebrofenin, without affecting overall hepatic exposure or biliary recovery. Simulations suggested that clinical doses of ritonavir may inhibit OATP-mediated hepatic uptake but not the MRP2-mediated biliary excretion, thus pinpointing the corresponding risk of transporter-mediated DDIs and the consequences at the organ and hepatocyte level (20).
The possibility that DDIs selectively occur at the level of canalicular hepatocyte transporters, with no impact on plasma pharmacokinetics but dramatic increases in liver exposure, has been confirmed with low-dose CsA using 99mTc-mebrofenin in rats (21). Such silent DDIs, which cannot be detected using conventional plasma pharmacokinetics, may result in increased hepatic accumulation of drugs. This situation is increasingly considered a mechanistic explanation for drug-induced liver injury, which is a major safety concern in drug development. Many drugs have an inhibitory effect on MRP2 in vitro (Table 1), and 99mTc-mebrofenin imaging may be used in drug development to predict the corresponding risk of pharmacokinetic DDIs in humans (33). These new applications extend the scope of this widely available molecular imaging probe beyond classic hepatobiliary scintigraphy to explore hepatocyte transporter function in health and disease (Table 2).
Compared with PET, γ-scintigraphy is traditionally considered a poorly quantitative imaging modality. Liver scintigraphy typically involves dynamic acquisitions with single-head 2-dimensional γ-cameras, which enables capturing the rapid uptake and biliary excretion of 99mTc-mebrofenin (4). However, such dynamic planar images lack the ability to assess liver function at the segmental level, therefore requiring the assumption of homogeneous hepatic function (14). With the introduction of hybrid SPECT/CT systems, there have been significant advances in image reconstruction with sophisticated compensation techniques, which now enable correction for photon attenuation and scattering. Quantitative SPECT/CT data can now be obtained in accurately delineated liver volumes and segments, although with low temporal resolution as compared with PET or γ-scintigraphy (34). Detailed knowledge regarding the carrier-mediated systems that control the liver kinetics of 99mTc-mebrofenin may be used to improve the pharmacokinetic properties of this radiopharmaceutical with respect to tomographic acquisition. In rats, blocking of the biliary excretion of 99mTc-mebrofenin was achieved by targeted inhibition of MPR2 using low-dose CsA, which prolonged the liver uptake phase and enhanced the liver exposure to 99mTc-mebrofenin (21). Since CsA is a clinically approved drug, these optimized conditions may potentially be applied in a clinical setting for improved assessment of functional liver volume with 99mTc-mebrofenin and SPECT (Table 2).
CONCLUSION
The discovery and characterization of different hepatocyte transporters have shed new light on the molecular determinants of the liver, bile, and plasma kinetics of 99mTc-mebrofenin. These properties enlarged the perspectives of this radiopharmaceutical agent to be repurposed for translational molecular imaging of liver transporter function. Enhanced knowledge of the liver transport of 99mTc-mebrofenin offers a molecular reading of scintigraphy data in various clinical situations with impaired liver function.
Future Perspectives of 99mTc-Mebrofenin for Clinical Applications
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank Thomas Beyer (Medical University of Vienna) for critically assessing this article.
Footnotes
Published online March 5, 2021.
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 15, 2020.
- Accepted for publication March 1, 2021.