Visual Abstract
Abstract
Liver cancer is a leading cause of cancer deaths worldwide. Surgical resection of superficial hepatic lesions is increasingly guided by the disrupted bile excretion of the fluorescent dye indocyanine green (ICG). To extend this approach to deeper lesions, a dedicated bimodal tracer that facilitates both fluorescence guidance and radioguidance was developed. Methods: A tracer comprising a methylated cyanine-5 (Cy5) fluorescent dye and a mercaptoacetyltriserine chelate (hHEPATO-Cy5) was synthesized and characterized. Cellular uptake and excretion were evaluated in hepatocyte cultures (2-dimensional culture and in vitro lesion model), using a fluorescent bile salt, MitoTracker dye, and methylated Cy5 as a control. After radiolabeling, the pharmacokinetics of 99mTc-hHEPATO-Cy5 were assessed in mice over 24 h (percentage injected dose and percentage injected dose per gram of tissue, SPECT/CT imaging and fluorescence imaging). The ability to provide real-time fluorescence guidance during robot-assisted hepatobiliary surgery was evaluated in a porcine model using ICG as a reference. Results: The unique molecular signature of hHEPATO-Cy5 promotes hepatobiliary excretion. In vitro studies on hepatocytes showed that where methylated Cy5 remained internalized, hHEPATO-Cy5 showed fast clearance (10 min) similar to that of fluorescent bile salt. In vivo use of 99mTc-hHEPATO-Cy5 in mice revealed liver accumulation and rapid biliary clearance. The effectiveness of bile clearance was best exemplified by the 2-orders-of-magnitude reduction in count rate for the gallbladder (P = 0.008) over time. During hepatobiliary surgery in a porcine model, hHEPATO-Cy5 enabled fluorescence-based lesion identification comparable to that of ICG. Conclusion: The bimodal 99mTc-hHEPATO-Cy5 provides an effective means to identify liver lesions. Uniquely, it helps overcome the shortcomings of fluorescence-only approaches by allowing for an extension to in-depth radioguidance.
- image-guided surgery
- fluorescence imaging
- hepatobiliary surgery
- bimodal tracer
- minimally invasive surgery
Annually, liver cancer accounts for an estimated 748,300 new cases and 695,900 cancer deaths worldwide. Next to primary liver cancer (e.g., hepatocellular carcinoma), tumorous lesions in the liver are often metastases of cancer with a different origin, such as colorectal cancer, neuroendocrine tumors, ocular melanoma, and breast cancer (1–5).
For both primary and metastatic liver cancer, surgical resection is considered essential to ensure long-term survival and to achieve a potential cure. The improvement in diagnostic imaging, the effectiveness of neoadjuvant systemic therapies, and the development of local treatment strategies has led to a 20%–30% increase in patients who are considered eligible for de novo surgery or surgery after neoadjuvant treatment (6). The success of these surgeries depends on the ability to achieve radical resection with preservation of normal liver tissue function (7). The high chance of hepatic recurrence (≤29% (8)) and complications (60% of cases (9)) indicates that substantial improvements can still be made in this line of therapy.
Accurate preoperative lesion identification, procedural planning, and intraoperative image guidance are of vital importance for precision surgery. Whereas preoperative lesion identification generally occurs via MRI, CT, or 18F-FDG PET (8), intraoperative identification often relies on the limited sensitivity and resolution of palpation, optical inspection, and intraoperative ultrasound (7,9). With the shift from open surgery to minimally invasive laparoscopic and robotic surgery (10), the reliance on image guidance technologies has increased. Especially, the use of fluorescence guidance is gaining ground. In 2009, Ishizawa et al. started to exploit the pharmacologic clearance profile of the fluorescent dye indocyanine green (ICG) to identify hepatic lesions with a high spatial resolution (11). Since then, this image guidance approach has been widely adopted, resulting in lower complications (odds ratio, 0.523) and shorter hospital stays (weighted mean difference, −1.8 (12)). A downside of the ICG-guided approach is that lesions located more than 5 mm below the surface cannot be reliably identified (9). Conversely, the use of separate techniques for pre- and intraoperative imaging can cause misalignment between the two (13). This problem not only limits planning and logistics but may also result in excision of additional (i.e., false-positive) tissue. Ideally, nuclear medicine diagnostics and intraoperative fluorescence guidance are integrated. In nuclear medicine, there are several radiotracers available that can be used to assess liver function (e.g., iminodiacetic acid (14) and 99mTc-mebrofenin (15)). However, to support approaches that combine noninvasive nuclear and intraoperative fluorescence imaging, there is a need for new bimodal or hybrid biliary tracers that contain both a radiolabel and a fluorescent label. Such a combined approach has already demonstrated clinical value in another surgical indication, that is, sentinel node resection (13).
Although the exact mechanism of ICG accumulation in liver lesions remains unclear, the fluorescence signal has been shown to accumulate at the transition between healthy and diseased tissue (9). In particular, disruptions in biliary clearance of the tracer are thought to play an important role in the localization of fluorescence in diseased tissues (16). With this feature in mind, we designed a small-molecule hybrid tracer to support bimodal imaging of liver lesions. After tracer synthesis, uptake and excretion were evaluated in vitro in hepatocytes, and in vivo tracer pharmacokinetics were assessed in mice. The surgical utility of the tracer was assessed during robot-assisted fluorescence-based hepatobiliary surgery in a porcine model.
MATERIALS AND METHODS
Synthesis and Chemical Evaluation of Hybrid Tracer hHEPATO-Cy5
Mercaptoacetyltriserine (mas3), N-Boc-aminophenol-Merrifield resin, and methyl-cyanine-5 (Cy5)-NH2 (also used as a Cy5 control) were synthesized according to previously described procedures (17,18). Methyl-Cy5-mas3 (hHEPATO-Cy5) (Fig. 1; Supplemental Figs. 1–4; supplemental materials are available at http://jnm.snmjournals.org) was synthesized as follows: mas3 (27 mg, 68 μmol), hexafluorophosphate azabenzotriazole tetramethyl uronium (26 mg, 68 μmol), and N-methylmorpholine (34 mg, 340 μmol) were dissolved in dimethyl sulfoxide (2 mL). Methyl-Cy5-amineC4 (30 mg, 68 μmol) was added, and the reaction mixture was stirred at room temperature for 25 min. A mixture of H2O/MeCN (85%/15%, 8 mL) with 0.1% trifluoroacetic acid was then added, and the crude product was purified through preparative high-performance liquid chromatography. Lyophilization yielded the product as a vibrant blue solid (30 mg, 54% yield). Compound characterization, including nuclear magnetic resonance (Supplemental Figs. 1–2), proton MR spectroscopy (Supplemental Fig. 3), high-performance liquid chromatography (Supplemental Fig. 4), absorption and emission (Supplemental Fig. 5), and brightness and serum protein binding, was performed as previously described (17).
Reaction scheme for synthesis of hHEPATO-Cy5: (a) methyl iodide, K2CO3, dimethylformamide; (b) N-(4-bromobutyl)phthalimide, sulfolane; (c) first, malonaldehyde dianilide HCl, 1:1 Ac2O:AcOH, followed by indole-amineC4Phth, 3:1 pyridine:Ac2O; (d) 33 wt % MeNH2 in EtOH; (e) l-mas3, hexafluorophosphate azabenzotriazole tetramethyl uronium, N,N-diisopropylethylamine.
In Vitro Tracer Metabolism in Hepatocytes
HC04 hepatocyte (19) and GEB3 epithelial control (20) cells were cultured in Gibco minimum essential medium enriched with 10% fetal bovine serum and penicillin/streptomycin (all Life Technologies Inc.). Three days before fluorescence confocal imaging, cells were seeded onto glass-bottom culture dishes (MatTek Corp.). An in vitro model for hepatic lesions was created by placing a heated metal rod in the center of the culture dish for 1–2 s, 1 h before imaging.
Samples were stained with 1 μM hHEPATO-Cy5 or 100 nM Cy5 control for 10 or 30 min at 37°C (6 samples per tracer and condition). Staining with 1 μM of the bile salt cholyl-Lys-fluorescein (21) or MitoTracker green (2 μL/mL M7514; Thermo Fisher) was used to confirm the staining pattern of hHEPATO-Cy5. Hoechst stain (33342, 1 mg/mL; Thermo Fisher) was added to all samples as a nuclear reference. Before imaging, samples were washed 3 times with phosphate-buffered saline.
Fluorescence confocal microscopy was performed as previously described using a Leica SP8 WL at sequential settings (22). Images were analyzed using the accompanying confocal software (LAS X; Leica Microsystems). Color selection was matched to the emission profile of the dye (Hoechst stain, 420–270 nm in blue; cholyl-Lys-fluorescein and MitoTracker, 500–550 nm in green; Cy5, 650–700 nm in red), using the color options in the LAS X software. For visualization of in vitro lesions, a tile scan consisting of a 5 × 5 grid at ×20 magnification was made. Semiquantitative image analysis and 3-dimensional surface plotting were performed using Fiji software as previously described (23).
Frozen excised tissue samples that contained a liver lesion were cut into 5-μm sections and imaged without further pretreatment. Additional sections were cut for standard hematoxylin and eosin staining, which was performed as previously described (24) and served as a reference for tissue morphology.
Animal Experiments
All rodent experiments were granted a license by the competent authority after receiving approval from the Animal Experiments Committee Leiden (AVD1160020173304). Experiments on pigs were approved by the ethical board of the University of Ghent (EC2019/79). Experiments were performed in an establishment licensed for the use of experimental animals (Leiden University Medical Center or Orsi Academy). Experiments were performed in accordance with the Experiments on Animals Act (2014), which is the applicable legislation in The Netherlands and Belgium, in accordance with the European guidelines (European Union directive 2010/63/EU) regarding the protection of animals used for scientific purposes.
Pigs were housed at the animal facility at Orsi Academy until used for imaging experiments during surgical training (weight per animal, ∼40 kg). Pigs were reused after surgical training and remained under anesthesia until being euthanized when the examination was completed.
In Vivo Tracer Biodistribution in Mice
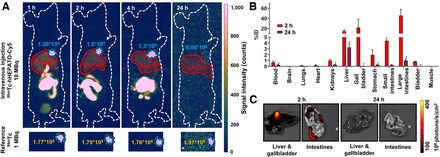
Radiolabeling of hHEPATO-Cy5 resulted in 99mTc-hHEPATO-Cy5 (high-performance liquid chromatograms are shown in Supplemental Fig. 4), and in vivo SPECT imaging (at 1, 2, 4, and 24 h) was performed according to previously described procedures (17). Reconstructions were visualized and analyzed with a custom MATLAB script (The MathWorks Inc.) that included correction for radioactive decay at the respective time point. Maximum-intensity projections with batlow color maps (25) were chosen, using identical color scaling at all time points for ease of comparison. Using the 2-dimensional scintigrams, we drew regions of interest over the gallbladder and liver for semiquantitative comparison of tracer uptake at every time point (decay-corrected). To reference the amount of radioactivity in these regions of interest, 1 MBq 99mTc point sources were imaged and analyzed under identical settings.
For quantitative assessment of the biodistribution of (hot) 99mTc-hHEPATO-Cy5, 10 MBq (0.5 nmol in 0.1 mL) of the labeled tracer were intravenously administered into female Swiss OF1 mice (6–7 wk old; Charles River). The percentage injected dose (%ID) and %ID per gram of tissue (%ID/g) were assessed at 2 and 24 h after intravenous tracer administration, as previously described (n = 9 (20,26)).
Semiquantitative assessment of fluorescence in the liver or gallbladder and intestines was assessed before γ-counting, to exemplify the effective hepatobiliary clearance and to complement the quantitative biodistribution, using an IVIS Spectrum preclinical imaging system (Perkin Elmer) and Living Image software (version 3.2 (26)). Images were acquired after excitation at 640 nm, and light was collected at more than 680 nm (acquisition time, 5 s). The fluorescent content was measured in photons/s/cm2. Because of the limited choice in color mapping in the commercial IVIS software, a hot color map was selected.
In Vivo Pharmacokinetic Assessments in Porcine Model: Surgical Fluorescence Imaging
Since porcine models do not naturally yield liver metastases, superficial heat-induced necrotic lesions were created using bipolar robotic instruments (da Vinci Maryland or fenestrated forceps [Intuitive Surgical]). These lesions served as a model for lesions that disrupt the hepatic anatomy.
A 3.75-mg quantity of hHEPATO-Cy5 was dissolved in 150 μL of ethanol, after which 1,350 μL of polysorbate 80 and saline were added to achieve a 2.5 mg/mL solution, which was subsequently intravenously injected into individual animals (n = 6). At 4–6 h after tracer administration, intraoperative imaging using both white light and Cy5 filtered light was performed using a modified clinical-grade IMAGE1 S camera system including a D-Light P light source (integrated customized Cy5 filter) and a customized 10 mm 0° laparoscope (Karl Storz SE & Co. KG (27)). The use of radiotracers and a more extended time window could not be pursued under the available ethical approval for the surgical training setting.
As a reference, identical experiments were conducted after administration of a similar dye concentration of ICG (3.75 mg, n = 2). In those experiments, imaging was performed using the fluorescence setting of a Firefly camera mounted on a Da Vinci surgical robot. The Da Vinci vision cart depicts ICG fluorescence as green on a black-and-white background without any form of scale bar. After imaging, the animals were euthanized, and lesions were excised for ex vivo fluorescence imaging and pathologic examination. To facilitate appreciation of the tracer uptake in these models, in-house–developed image processing was applied, using custom algorithms written in MATLAB. The fluorescence signal was segmented on the basis of color and visualized as an overlay on a black-and-white representation of the anatomy. Regions of interest were drawn on healthy liver tissue for background fluorescence assessments. The median fluorescence background signal was used to calculate the signal-to-background ratio for each fluorescent pixel. The signal-to-background ratios were depicted using a batlow color map with scale bar (25).
Statistical Analysis
Statistical evaluation to compare uptake values (%ID and %ID/g for radioactive assessment and photons/s/cm2 for the fluorescence signal) at different time points in the biodistribution was performed using an unpaired 2-sided Student t test. Values of P that were less than 0.05 were considered significant.
RESULTS
Synthesis and Characterization
hHEPATO-Cy5 was successfully synthesized (Fig. 1; Supplemental Figs. 1–4) and presented favorable chemical properties (serum protein binding, 94%; logP, 0.80 ± 0.03) and fluorescent properties (absorption/emission, 640/665 nm [Supplemental Fig. 5]; brightness, 3,445 M−1). After radiolabeling,99mTc-hHEPATO-Cy5 was obtained with a radiochemical yield of 83% ± 5%.
In Vitro Excretion Hepatocytes
Using an in vitro lesion model and assessment in hepatocyte cultures, the difference in uptake of hHEPATO-Cy5 in damaged and healthy hepatocytes was compared (Supplemental Fig. 6; Fig. 2). In the in vitro lesion model, a high-intensity Cy5 fluorescence signal was detected in a ring of damaged hepatocytes (Supplemental Fig. 6). In these cells, uptake was distributed evenly over the whole cell. A change in distribution of the fluorescence signal was seen with increasing distance from the lesion. At an approximately 500-μm distance, the overall uptake pattern was like that in healthy hepatocytes. Here, no intracellular uptake was seen, but focalized uptake of hHEPATO-Cy5 was positioned in bile cannulas between cells (Fig. 2; Supplemental Fig. 6 (28)). As this pattern was shown to be similar to that of fluorescent bile salts (Fig. 2), this suggests that hHEPATO-Cy5 is functionally excreted.
Fluorescence confocal imaging of HC04 hepatocytes after 10 min of incubation with fluorescent bile salt cholyl-Lys-fluorescein or coincubation of hHEPATO-Cy5 and cholyl-Lys-fluorescein), Cy5 control, or MitoTracker. Left images in each panel are 2-dimensional fluorescence confocal images (LAS X software). Right images in each panel are 3-dimensional representations of tracer distribution throughout cell (Fiji software). Nuclear staining is in blue, Cy5 is in red, and MitoTracker is in green. Dashed circle is example of bile canula, and yellow lines are orientation 3-dimensional analysis. Color bars show pixel intensity (arbitrary units) obtained with same software. AU = arbitrary units; CLF = cholyl-Lys-fluorescein; T = time after incubation.
Incubation of epithelial cells with hHEPATO-Cy5 resulted in mitochondrial uptake (Supplemental Fig. 7), which was similar to the uptake of the fluorescent component alone (methylated Cy5 [control]) in both hepatocytes and epithelial cells (Fig. 2; Supplemental Fig. 7). Excretion of hHEPATO-Cy5 was underlined by semiquantitative assessment of the fluorescence signal over time (Supplemental Fig. 8). In healthy hepatocytes, a decrease in hHEPATO-Cy5–related fluorescence signal was seen after 30 min. In contrast, a substantially higher (P > 0.0001) intracellular signal that did not decrease over time was seen for the Cy5 control. Hence, the focal uptake of hHEPATO-Cy5 is exclusive to hepatocytes, and the addition of the mas3 moiety in hHEPATO-Cy5 is crucial for the hepatobiliary excretion.
In Vivo Biodistribution in Mice
As early as 1 h after intravenous administration, hot 99mTc-hHEPATO-Cy5 (0.5 nmol) yielded dominant hepatobiliary excretion, exemplified by the prominent signal seen in the liver, gallbladder, and intestines on SPECT (Fig. 3A). Biliary excretion was further substantiated by a significant 2-orders-of-magnitude reduction in count rate for the gallbladder (Fig. 3B; Supplemental Table 1; P = 0.008) over time. Instrumental for imaging of liver lesions, a 5-fold decrease in count rate was observed in the liver (Supplemental Table 1; P = 0.002) and was further substantiated via ex vivo tissue examination at 2 and 24 h (Fig. 3C). At 2 h after tracer injection, high fluorescence intensities were seen in the gallbladder (2.6 × 108 ± 5.9 × 107 photons/s/cm2), whereas intensities in the liver were significantly lower (3.3 × 107 ± 7.9 × 106 photons/s/cm2; P > 0.0001). After 24 h, fluorescence signal intensities in the liver had further reduced to 1.6 × 107 ± 2.8 × 106 photons/s/cm2, which correlated to a negligible background staining in this tissue (Fig. 3C).
Biodistribution of 99mTc-hHEPATO-Cy5 in mice. (A) In vivo SPECT imaging (batlow color map) at 1, 2, 4, and 24 h after tracer administration. Shown are decay-corrected signal intensity (counts) for liver and gallbladder (in red and blue regions of interest, respectively; top images) and for 99mTc reference source (bottom images). (B) Quantitative biodistribution presented as %ID at 2 and 24 h after intravenous administration in mice. (C) IVIS fluorescence imaging of liver and gallbladder and intestines using hot map (Living Image Software [Perkin Elmer]; photons/s/cm2) at 2 and 24 h. *P > 0.01 for 2- vs. 24-h uptake values.
In Vivo Pharmacokinetic Assessments in Porcine Models: Surgical Fluorescence Imaging
In vivo imaging in a porcine model for robot-assisted hepatobiliary lesion resection was performed to show the similarities in staining pattern with the current clinical fluorescence-only approach (using ICG) and compatibility of hHEPATO-Cy5 with clinical-grade robotic surgery and imaging devices. Fluorescence imaging in this model underscored the hepatobiliary clearance profile for hHEPATO-Cy5. A clear fluorescence signal was seen in the gallbladder and intestines. And importantly, at 4 h after injection, the background uptake in the nonaffected liver was already negligible (Fig. 4).
Surgical imaging setup in porcine model. Use of clinical-grade equipment allowed combination of robot-assisted surgery and laparoscopic fluorescence imaging (22) for assessment of tracer distribution over hepatobiliary system and excretion toward intestines. At center top is schematic localization of organs of interest, and at center bottom is batlow laparoscopic fluorescence image showing signal-to-background ratios of hHEPATO-Cy5-related fluorescence. Dashed circle represents area used to define background signal. # = gallbladder; ## = liver; ### = intestines; SBR = signal-to-background ratio.
In vivo created liver lesions (Fig. 5) demonstrated a characteristic fluorescent rim around the border of the lesion (n > 25 lesions tested). Clear discrimination could be made between the liver and surrounding tissue of the abdominal wall, and this discrimination was especially evident for lesions on the outer edge of a liver segment. The rimlike staining pattern was comparable to the accumulation seen in the in vitro model (Supplemental Fig. 6) and in line with that of ICG in the same model (Supplemental Fig. 9) but also of ICG in patients with liver cancer (16).
Robot-assisted in vivo imaging of liver lesions in porcine model. (A) Schematic representation of creation of heat-induced lesions using coagulation setting of bipolar robotic forceps. (B and C) In vivo laparoscopic imaging of liver lesions (arrows) and surrounding tissue of abdominal wall (*) using white-light imaging (B) and custom image-processing algorithms (C) support batlow intensity-based assessment of fluorescence uptake. Dashed circle is area used to define background signal. SBR = signal-to-background ratio (27).
Microscopic pathologic examination of the excised lesions underscored the difference in morphology between the healthy liver tissue and the lesion (Figs. 6A and 6B). Accumulation of hHEPATO-Cy5 occurred within a transitional rim bridging the lesion and healthy liver tissue (Fig. 6C). This suggests that damaged hepatocytes in the transitional rim are unable to facilitate bile transport, resulting in local retention of hHEPATO-Cy5.
Localization fluorescence signal in liver lesions. (A) Immunohistochemistry (hematoxylin and eosin staining) of excised liver sample containing healthy liver tissue (**) and heat-induced liver lesion (encircled). (B and C) Zoomed area showing transitional rim (between dashed lines) between necrotic liver tissue (#) and healthy liver tissue (B) and fluorescence confocal imaging in sequential tissue section with fluorescence uptake (in red [Leica LAS X software]) in transitional rim (C). Color bar shows pixel intensity (arbitrary units) obtained with same software. AU = arbitrary units.
DISCUSSION
By directly conjugating a lipophilic Cy5 dye to a mas3 chelate, we generated a hybrid hepatic tracer (hHEPATO-Cy5). This tracer portrays a unique biliary excretion profile (Fig. 2; Supplemental Figs. 7 and 9) in hepatobiliary cultures. In vivo, the tracer allowed reliable delineation of liver lesions in real time using fluorescence-guided robot-assisted surgery in a porcine model (Fig. 5).
The hybrid nature of 99mTc-hHEPATO-Cy5 extends fluorescence imaging by giving it the ability to identify preoperative lesions (SPECT). In other oncologic surgical applications, this combination has been shown to provide value that is greater than the sum of the benefit of the individual techniques (29). Preoperative knowledge of the exact location of the lesion before tissue exploration, along with visual assessment and validation of the excision of the targeted tissue, is a feature likely to provide steps toward overcoming existing challenges in hepatobiliary surgery.
Although the correlation between nuclear and fluorescence imaging based on hHEPATO-Cy5 was shown in mice, constraints within the ethical license prohibited use of radioactivity in the porcine model. As such, in the latter model, use of only the fluorescent readout and subsequent assessment of superficial lesions was allowed. Nevertheless, the literature indicates that intraoperative extension of fluorescence imaging with in-depth drop-in radioguidance (30) is likely to help facilitate the resection of deeper lesions. This assumption is supported by studies in other clinical indications that provide clear evidence of the superior in-depth image guidance that can be achieved when a hybrid tracer is used rather than a fluorescence-only tracer (31).
The mechanism behind ICG uptake in hepatobiliary lesions remains a subject of study. Our work clearly indicates that uptake of hHEPATO-Cy5 around a lesion can be attributed to disrupted hepatocytes and is related to the excretion of bile (Fig. 2; Supplemental Fig. 6). Further mechanistic studies are needed to help understand if this relates to specific transporter proteins (16).
Fluorescent emissions used for image-guided surgery are classified into 3 categories according to the International Union of Pure and Applied Chemistry regulations: near-infrared, far-red, and visible fluorescence (31). Despite the popular demand for near-infrared analogs (e.g., ICG; maximum emission wavelength, 750–1,000 nm), there are clear arguments to be made for the surgical use of far-red dyes (e.g., Cy5; maximum emission wavelength, 650–750 nm). For example, previous investigations have shown that Cy5 has better photophysical properties than ICG (32) and that clinical systems have higher sensitivity to Cy5 than to ICG, both aiding deeper detection. Surprisingly, Cy5 is quite often falsely referred to as being a near-infrared dye (24,33). Using a range of clinical-grade Cy5 camera prototypes has resulted in the successful in-patient use of far-red dyes (24,27,34). The depiction of Cy5 fluorescence in vivo (Fig. 5) has even been shown to be compatible with imaging of near-infrared dyes (in the same patient), allowing unique multispectral imaging strategies (32,35).
Creation of relevant in vivo large-animal models for resection of hepatic lesions is not trivial. To the best of our knowledge, this is the first report of such a model that can be used to evaluate image guidance technologies for hepatobiliary lesions. The lesions displayed ICG uptake that is in line with literature reports (16,36). Clinical follow-up studies will be required to confirm the translational value of hHEPATO-Cy5.
CONCLUSION
By creating a small molecule comprising a fluorescent Cy5 dye and a mas3 chelate, we have designed a unique bile-excreted hybrid tracer—one that is capable of providing both fluorescence guidance and radioguidance during excision of liver lesions. The hybrid nature of this tracer also paves the way for the future implementation of nuclear medicine road maps to plan and guide fluorescence-based hepatobiliary surgery.
DISCLOSURE
This research was funded by an NWO-TTW-VICI (TTW BGT16141) grant supported by the Dutch Research Council. Julia Kloetzl is an employee of Karl Storz SE & Co. KG. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is it possible to design a hybrid radiolabeled and fluorescently labeled tracer that allows visualization of liver lesions in a manner similar to the clinical application of the fluorescent dye ICG?
PERTINENT FINDINGS: The unique molecular composition of the tracer 99mTc-hHEPATO-Cy5 promotes hepatobiliary excretion via a mechanism like that of bile salts. The hybrid nature of the tracer means it allows both in vivo SPECT of hepatic clearance in mice and fluorescence-guided liver lesion resection in a porcine model.
IMPLICATIONS FOR PATIENT CARE: The hybrid nature of 99mTc-hHEPATO-Cy5 helps extend the current clinically applied fluorescence-only approach to one that supports in-depth target identification and one that can be supported by road maps generated through preoperative tracer imaging.
ACKNOWLEDGMENTS
We thank Danny van Willigen and Thom van den Eng for their contributions to tracer synthesis, evaluation, and in vitro analysis.
Footnotes
↵* Contributed equally to this work.
Published online Jul. 18, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication September 25, 2023.
- Accepted for publication May 28, 2024.