Visual Abstract
Abstract
Quantitative imaging biomarkers are widely used in PET for both research and clinical applications, yet bias in the underlying image data has not been well characterized. In the absence of a readily available reference standard for in vivo quantification, bias in PET images has been inferred using physical phantoms, even though arrangements of this sort provide only a poor approximation of the imaging environment in real patient examinations. In this study, we used data acquired from patient volunteers to assess PET quantitative bias in vivo. Image-derived radioactivity concentrations in the descending aorta were compared with blood samples counted on a calibrated γ-counter. Methods: Ten patients with prostate cancer were studied using 2-(3-(1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid PET/CT. For each patient, 3 whole-body PET/CT image series were acquired after a single administration of the radiotracer: shortly after injection as well as approximately 1 and 4 h later. Venous blood samples were obtained at 8 time points over an 8-h period, and whole blood was counted on a NaI γ-counter. A 10-mm-diameter, 20-mm-long cylindric volume of interest was positioned in the descending thoracic aorta to estimate the PET-derived radioactivity concentration in blood. A triexponential function was fit to the γ-counter blood data and used to estimate the radioactivity concentration at the time of each PET acquisition. Results: The PET-derived and γ-counter–derived radioactivity concentrations were linearly related, with an R 2 of 0.985, over a range of relevant radioactivity concentrations. The mean difference between the PET and γ-counter data was 4.8% ± 8.6%, with the PET measurements tending to be greater. Conclusion: Human image data acquired on a conventional whole-body PET/CT system with a typical clinical protocol differed by an average of around 5% from blood samples counted on a calibrated γ-counter. This bias may be partly attributable to residual uncorrected scatter or attenuation correction error. These data offer an opportunity for the assessment of PET bias in vivo and provide additional support for the use of quantitative imaging biomarkers.
PET has a rich history of quantitative methods (1). Although quantitative imaging is a relatively recent phenomenon in other areas of radiology (2), PET has been quantitative from its inception. Studies of neuroreceptor binding, myocardial perfusion, and tumor response assessment are just a few examples of the many quantitative applications of PET. Quantitative data are usually regarded as being less subjective than qualitative image assessment and are readily amenable to statistical analysis. They allow for graded characterization of processes that might not fall into a binary classification. And in some cases, quantification provides information that is not discernible by visual inspection alone, such as myocardial perfusion studies in cases of balanced ischemia (3). For these reasons, quantitative methods not only are pervasive in PET research but also have been widely incorporated into routine clinical imaging, where parameters such as SUVs are extensively used.
PET allows for a wide range of quantitative techniques with different levels of complexity ranging from simple SUV to elaborate dynamic studies incorporating blood analysis and radiotracer kinetic modeling (4). In general, quantification is feasible because all key steps in PET image formation are linear (or approximately linear) and most of the effects that degrade the measured data can be corrected or minimized. Although spatial resolution varies slightly across the field of view, PET images are generally free from gross distortions. Sophisticated corrections for detector nonuniformity, geometric issues, detector dead time, random coincidences, scatter, and attenuation have been meticulously developed (5). If all things work as intended, the absolute value of the image voxels should have some meaning. Most PET-based biomarkers assume that the voxel intensity reflects the local radioactivity concentration, which in turn reflects the properties of the radiopharmaceutical, the time since radiotracer administration, and, most importantly, the characteristics of the patient. Surprisingly, the assumption that PET images reflect radioactivity concentration within the body has not been widely tested. A common concern is that quantitative measurements are not directly comparable between different scanners (6), even for regions that are not greatly affected by partial-volume issues. Of course, a wealth of experience supports the assumption that PET images do reflect radioactivity concentration, but the accuracy (strictly bias) with which radioactivity concentration can be quantified within the body is less clear.
A likely reason for the lack of data assessing bias for in vivo quantification is that the true radioactivity concentration within the body is generally not known. Extensive research has evaluated all aspects of the quantitative process, but these evaluations have had to use surrogate markers such as phantoms (7) or computer simulations (8). Computer simulation allows fine control of the relevant parameters and the ability to compare measurements with ground truth. Although these methods and results are convincing, there is a sense that the evaluations are not quite complete without data acquired on real scanners. In the absence of a readily available reference standard for in vivo quantification, the accuracy of PET images has had to be inferred using physical phantoms, even though arrangements of this sort provide only a poor approximation of the imaging environment in patient studies. Phantom-based evaluations may give an unrealistically optimistic impression of PET quantitative accuracy because they do not reflect the complexity of the scatter and attenuation distributions in real patients. In the present study, we used data acquired from patient volunteers to assess PET quantitative accuracy in vivo. Image-derived radioactivity concentrations in the descending aorta were compared with blood samples counted on a calibrated γ-counter. In this way, we were able to compare in vivo PET measurements with a reliable external reference.
MATERIALS AND METHODS
Data Acquisition
This prospective study was approved by the Johns Hopkins institutional review board, and all participants provided written informed consent. Ten patients with prostate cancer were studied using the prostate-specific membrane antigen PET imaging agent 2-(3-(1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid (18F-DCFPyL) (9) as part of the larger OSPREY phase II/III clinical trial (ClinicalTrials.gov identifier NCT02981368). Each patient had 3 whole-body PET/CT scans after a single administration of approximately 333 MBq (9 mCi) of 18F-DCFPyL. PET acquisitions took place shortly after radiotracer injection and approximately 1 and 4 h later. Images were acquired from mid thigh to skull vertex using a Biograph mCT (Siemens Healthineers), which had a 21.8-cm axial field of view (10). Whole-body acquisition used a step-and-shoot approach for 3 min per bed position, with adjacent bed positions overlapping by 10 cm. Patients were allowed to get off the PET/CT bed after each whole-body study, and separate CT scans were acquired at each of the 3 imaging sessions (120 kV, 64 mA, 0.8 pitch, 0.5-s rotation time).
PET images were reconstructed using 3-dimensional ordered-subsets expectation maximization with time-of-flight technique, 2 iterations, 21 subsets, and a gaussian filter of 5 mm in full width at half maximum (FWHM). Standard corrections were applied for dead time, randoms (offline subtraction of delayed events), attenuation (CT-based), and scatter (single scatter simulation, with scatter estimates scaled to the tails of the projections). Resolution recovery was not incorporated into the reconstruction algorithm. Image voxel dimensions were 4 × 4 mm and had a 3.3-mm slice thickness. The system was calibrated according to manufacturer recommendations to ensure that PET images were generated in units of Bq/mL. This process involved a combination of daily normalization and calibration adjustment with a 68Ge phantom and annual cross-calibration using an 18F-filled, 20-cm-diameter uniform cylinder phantom. The 18F procedure was performed after replacement of the 68Ge phantom and served to cross-calibrate the scanner to the local dose calibrator. Both phantom and patient 18F activities were measured with the same dose calibrator (CRC 15W; Capintec) and geometry (including syringe type and sample holder), using a calibration factor of 447. This calibration factor was derived specifically for this instrument using a 68Ge standard source (BM06S-68; Radqual Global Sources), traceable to the National Institute of Standards and Technology, which had itself been cross-calibrated for 18F. The locally derived factor was identical to a previously published value for 18F with this same model of instrument (11).
Blood samples were obtained from an indwelling venous catheter at 8 time points over an 8-h period, approximately 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, and 8 h after radiotracer injection. Samples of whole blood (300 μL each) were counted on a Wizard 2480 (Perkin Elmer) γ-counter (12). The counting protocol used a 409- to 613-keV energy window and acquired data for 60 s per sample. The efficiency of the γ-counter for 18F (32.7%) was experimentally determined using the dose calibrator described above. This calibration procedure involved 300-μL samples of an 18F stock solution, counted using the same protocol, test tubes, and sample geometry as used for the patient blood samples. Application of this efficiency factor enabled the γ-counter to measure 18F radioactivity and therefore radioactivity concentration in absolute terms (Bq/mL). In this way, the dose calibrator, the PET/CT scanner, and the γ-counter were cross-calibrated with respect to one another and with reference to a national metrology laboratory.
Data Analysis
Radioactivity concentrations obtained from regional analysis of the PET images were compared with blood sample measurements as follows.
In each PET/CT image series, a cylindric volume of interest (VOI) was defined in the descending thoracic aorta to estimate the PET-derived radioactivity concentration in blood. These VOIs were 10 mm in diameter and 20 mm long and were automatically positioned using commercial software (Syngo Via; Siemens Healthineers). The mean radioactivity concentration of all voxels within the VOI was recorded, and the measurement time was taken to be the acquisition time of a slice passing through the region. Because adjacent bed positions in a multibed acquisition overlap each other, a single slice would typically be formed from data acquired at 2 different bed positions, in which case interpolation was used to estimate the acquisition time. VOI measurements from each of the 3 whole-body studies were decay-corrected back to radiotracer injection time.
Because blood sample collection times did not necessarily coincide with PET VOI measurement times, an analytic model was fit to the γ-counter data for each patient. γ-counter measurements were decay-corrected back to injection time and plotted as a function of sample collection time. A triexponential model was fit to the γ-counter data using least-squares minimization. The model was optimized for each patient and used to estimate the γ-counter–derived radioactivity concentration at the time of each PET measurement.
In this way, 3 pairs of data were generated for each patient, corresponding to the PET-derived (C
PET) and γ-counter–derived (C
γ) radioactivity concentrations at each scan. The extent of the agreement between the 2 methods was assessed using a Bland–Altman approach (13). The difference (d) between corresponding data points was calculated as Eq. 1and the relative difference (D) was calculated as
Eq. 1and the relative difference (D) was calculated as Eq. 2The difference data were plotted as a function of the mean, 0.5(C
PET + C
γ), and the Kendall tau was used to test for proportionality. When difference was dependent on the magnitude of the measurement, relative difference was used for subsequent analysis. If these data were consistent with the normal distribution (Shapiro–Wilk test), 95% limits of agreement were defined as
Eq. 2The difference data were plotted as a function of the mean, 0.5(C
PET + C
γ), and the Kendall tau was used to test for proportionality. When difference was dependent on the magnitude of the measurement, relative difference was used for subsequent analysis. If these data were consistent with the normal distribution (Shapiro–Wilk test), 95% limits of agreement were defined as  Eq. 3where mean(D) and SD(D) indicate the mean and SD of D, respectively. Statistical analysis was performed using SPSS Statistics, version 25 (IBM), and a P value of 0.05 was taken as the threshold for significance.
Eq. 3where mean(D) and SD(D) indicate the mean and SD of D, respectively. Statistical analysis was performed using SPSS Statistics, version 25 (IBM), and a P value of 0.05 was taken as the threshold for significance.
Phantom Experiments
Throughout the period of patient accrual, PET/CT phantom data were acquired quarterly. The purpose of these phantom studies was to monitor the accuracy of scanner calibration and to assess the partial-volume effect for a cylindric insert comparable in size to the descending thoracic aorta. The American College of Radiology PET phantom (14) was prepared with an aqueous solution of 18F and scanned with the same acquisition and reconstruction protocol as used for patient imaging. A VOI in a large, uniform region was used to measure quantitative accuracy, and an SUV of 1.0 would indicate perfect agreement with the expected value, based on the dose calibrator. SUV was calculated in this phantom compartment using the mass of water, which was measured using an accurate balance. In addition, a 25-mm-diameter cylindric insert within the phantom was filled with a known radioactivity concentration 2.5 times that of the background and used to assess the partial-volume effect. A 10-mm-diameter cylindric VOI was placed in the center of the insert, and the ratio of the mean VOI measurement to the known radioactivity concentration was defined as the recovery coefficient, expected to be 1.0 in cases of negligible partial-volume underestimation. This approach was intended to mirror patient image analysis in terms of the dimensions of the VOI and the size and shape of the descending thoracic aorta.
RESULTS
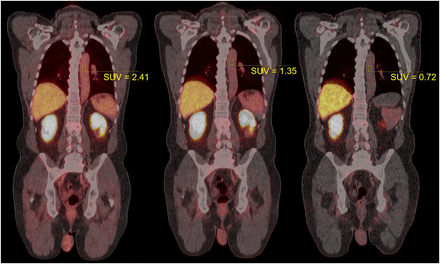
Between October 2017 and March 2018, 10 patients underwent 18F-DCFPyL PET/CT with data collection as described above. The mean injected activity was 337 ± 11 MBq. Figure 1 shows example images for a particular patient, with the position of the aorta VOIs clearly displayed. Figure 2 shows corresponding γ-counter measurements, the resulting triexponential model fit, and PET VOI measurements. No data were lost or compromised; in all, 30 pairs of quantitative measurements were available for statistical analysis.
Example PET/CT images for typical patient. PET data were acquired 24, 79, and 264 min after injection (from left to right). Cylindric VOIs can be seen in descending aorta, with SUV as indicated. Fused display shows PET in hot-body color map, scaled from 0 to 20 SUV units in each image. Patient weighed 90.5 kg and was administered 339.7 MBq of 18F-DCFPyL.
Example time–activity data for patient shown in Figure 1. Circles indicate whole blood samples counted on calibrated γ-counter. Squares indicate PET-derived data from VOIs in descending aorta. Line is triexponential model fit to γ-counter data. All data were decay-corrected to time of injection.
Figure 3 shows that the PET and γ-counter data were linearly related (R 2 = 0.985) over a range of relevant radioactivity concentrations. Figure 4A shows that the difference data were proportional to the mean (Kendall tau = 0.338, P = 0.009). However, when the data were expressed in relative units, the proportionality was not significant (Kendall tau = 0.149, P = 0.246). In other words, bias expressed in percentage form was approximately constant across a range of radioactivity concentrations (Fig. 4B). The mean bias (the mean relative difference over all patients and studies) was 4.8% ± 8.6%, with the PET measurements tending to be greater. These relative difference data were not normally distributed (Shapiro–Wilk test, P = 0.03). However, for a subset of the data in a clinically relevant range above 5,000 Bq/mL (corresponding to an SUV > 1 in a 74-kg patient after a 370-MBq administration), the relative difference data were normally distributed (Shapiro–Wilk test, P = 0.38). In this range, the mean bias was 5.2% ± 4.0%, with 95% limits of agreement of −2.6% and +13.0%.
PET and γ-counter radioactivity concentration data for 10 patients, each with 3 corresponding measurements. Line indicates linear fit to data: y = 1.06x – 64, R 2 = 0.985.
Bland–Altman plots showing difference between PET and γ-counter measurements in radioactivity concentration units (A) and relative units (B). Lines indicate linear regression. Relative difference was not proportional to radioactivity concentration and had mean value of 4.8% ± 8.6%.
Four phantom studies were performed around the study period. The SUVmean in the uniform region was 0.993 ± 0.005, indicating high accuracy and stability with this phantom arrangement. The mean recovery coefficient for the 25-mm insert was 1.002 ± 0.014, indicating negligible partial-volume underestimation for a 10-mm VOI and a cylindric object with a diameter of at least 25 mm. For comparison, the mean diameter of the descending thoracic aorta at the level of the VOI was 27.4 ± 2.4 mm, based on measurements from the 30 patient CT images.
DISCUSSION
In this study, we assessed the quantitative accuracy of PET images in real human studies. More specifically, we compared PET measurements of the radioactivity concentration in the descending thoracic aorta with blood samples counted on a γ-counter, carefully calibrated with reference to a national metrology laboratory. On average, the PET image data were 4.8% ± 8.6% greater than the γ-counter data. These results indicate the bias that should be expected in basic PET measurements and confirm the relatively high accuracy that can be achieved in human imaging.
Early work establishing the foundations for PET quantification of radioactivity concentration were based on phantom experiments (15) and subsequently supported by in vivo measurements in animals. Using a similar approach to the one used in the present study, PET and γ-counter blood measurements were shown to be highly correlated after partial-volume correction based on postmortem measurement of organ dimensions (16). The importance of partial-volume correction reflects the limited spatial resolution of the PET systems available at that time, estimated to be approximately 16 mm FWHM. In contrast, the modern PET system used in our work had an effective spatial resolution of approximately 7 mm FWHM (17), and the partial-volume effect was not expected to be a significant source of error in our aorta VOI measurements. This expectation was confirmed by phantom experiments that involved a 25-mm cylindric insert of approximately the same size as the descending aorta (27 mm) at the level of the VOI measurement. Note that the bias results reported in the present paper apply to large organs for which partial-volume errors can be neglected. It is worth emphasizing that much greater bias should be expected for smaller lesions, less than roughly 3 times the effective FWHM.
The current assessment of the quantitative accuracy of PET for in vivo human imaging is particularly relevant because it was performed on a modern PET/CT system. Modern scanners incorporate many components that could potentially degrade quantitative accuracy, including 3-dimensional data acquisition, scintillators with intrinsic radioactivity, iterative reconstruction, CT-based attenuation correction, and scatter correction models that include various assumptions. Although each aspect has been tested individually, the overall effect on quantitative accuracy has not been extensively studied, most likely because of the lack of a readily available reference standard for in vivo quantification. Here, we assumed that the radiotracer activity concentration in samples of whole blood from a vein in the arm was comparable to that of arterial blood in the aorta. Unlike the case with 18F-FDG, for which arterial and venous concentrations differ substantially as a function of time (18,19), this assumption is a very reasonable one for 18F-DCFPyL (20). In previous work by a separate group (21,22), radioactive urine samples were used as a reference for assessing bias in vivo. PET and PET/CT images were found to underestimate radioactivity concentration by 7%–12%. This bias is in the opposite direction to our own results and may be related to the extremely high radioactivity concentration in the bladder, possibly causing incomplete convergence of the iterative reconstruction algorithm and other problems.
The discrepancy of around 5% between our PET and γ-counter measurements could be due to a combination of various technical factors, although many of these sources of error are not expected to have a large impact. The phantom data indicated that partial volume is not likely to be a major effect. The related effect of signal overestimation due to proximity to other organs with a higher radioactivity concentration (spillover) is also expected to be negligible, because 18F-DCFPyL is not taken up to any great extent in nearby organs. Detector dead time was not expected to be a problem for either the PET scanner or the γ-counter, based on the measured count rates. The effect of respiratory motion was expected to be minimal because of the long extent of the aorta in the craniocaudal direction and, in any event, would not readily explain the overestimated PET measurements. A perhaps more significant source of error is the possibility of imperfect scatter correction. Scatter is a major problem in 3-dimensional PET, and in this study cohort the scatter correction algorithm indicated a scatter fraction of 38.6% ± 2.5% at the level of the descending aorta. Perfect compensation in software cannot be assumed, especially in the chest, where the scatter distribution is particularly complex and may have resulted in a residual uncorrected scatter component that increased the measured PET signal. Another possibility is that errors in the CT-based attenuation correction could also contribute to the observed bias. CT images are acquired at energies well below 511 keV and need to be scaled so as to reflect the attenuation appropriate for 511-keV annihilation radiation. Small errors in this process cannot be excluded, particularly as the chest includes a complex distribution of organs (bone, lung, and soft tissue) with very different attenuating properties.
It is worth noting similarities between the present work and previous studies that have used PET images to derive input functions for radiotracer kinetic modeling. In studies of that kind, the goal was to estimate the time course of radiotracer activity in arterial plasma without the need for invasive blood sampling. Correction for the partial-volume effect is essential for brain studies because the only available blood vessels in the field of view are small compared with the spatial resolution of the system. Although the need for partial-volume correction is clear, problems involved with successful application have also been noted (23). When imaging included the chest, larger blood vessels were available such as the aorta, which was often preferred over the left ventricular cavity because of spillover from the myocardial walls (24). Evaluation of these image-derived input functions did not necessarily involve direct comparison of the original image data with arterial blood samples counted on a γ-counter. For example, in many cases the image-derived data were scaled with reference to one or more blood samples (25,26) and therefore did not reflect the quantitative accuracy of the original image data. When input functions derived from unscaled PET data and arterial blood sampling were available for comparison, the assessment was commonly characterized in terms of the resulting kinetic parameters (24,27), as opposed to a direct comparison of radioactivity concentration estimates.
In the present study, we directly compared image- and sample-based measurements of blood radioactivity concentration. The purpose of this evaluation was to provide an estimate of the bias that can be expected in PET-derived quantitative metrics. Of course, some PET metrics such as metabolic tumor volume are not dependent on calibration accuracy because they reflect the volume, rather than the concentration, of radiotracer. Also if both the input function and the tissue time–activity data are derived from the same image, as is commonly the case with cardiac flow quantification, errors due to scanner miscalibration cancel out and the need for accurate cross-calibration of equipment is avoided. However, for many PET biomarkers, including SUV, accurate image calibration is critical. In the present study we used a γ-counter, calibrated to a national metrology laboratory, as a reference. Both PET and γ-counter measurements will have errors, but we expect the γ-counter to be accepted as a reliable reference method. These results are strictly applicable only to the radiotracer used in this study, 18F-DCFPyL. Although similar bias might be expected with other 18F-labeled radiotracers, more complex isotopes such as 124I or 82Rb would require further evaluation because of the presence of problematic prompt γ-rays. Different results might also be expected in other parts of the body, but we suspect that the chest may represent one of the more difficult settings because of its complex scatter and attenuation environment.
The results presented here have particular relevance for efforts to standardize quantitative PET biomarkers across different scanners and institutions (28). Although patient test–retest studies provide information on repeatability (29), bias has been harder to characterize in vivo. Here, we propose an approach that addresses this problem, allowing bias to be estimated directly from patient images. Furthermore, since the amount of radiotracer in blood was continuously changing, we were able to assess bias over a range of relevant radioactivity concentrations. The method is generally applicable to other PET/CT models, as well as to other PET devices. For example, the method may be particularly useful for evaluating combined PET/MR systems, for which the absence of linear attenuation coefficient measurements makes attenuation correction problematic (30). Using this method, we have shown that low bias is feasible with conventional clinical PET/CT scanners under normal operating conditions. Of course, our specific results reflect the performance of only one particular scanner system, and variability among different scanners and institutions is to be expected.
CONCLUSION
Human image data acquired on a conventional whole-body PET/CT system with a typical clinical protocol differed by an average of around 5% from blood samples counted on a calibrated γ-counter. This relatively low bias is encouraging, particularly as it was measured in the complex imaging environment encountered in the chest, and may be partly attributable to residual uncorrected scatter or attenuation correction error. These data offer an opportunity to assess PET bias in vivo and provide additional support for the use of quantitative imaging biomarkers.
DISCLOSURE
Martin Pomper is a coinventor on a U.S. patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. Steven Rowe and Martin Pomper have received research funding from Progenics Pharmaceuticals. This work was supported by a grant from Progenics Pharmaceuticals, Inc., and by R01-CA134675. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: PET quantitative metrics such as SUV assume that image voxels reflect the local radioactivity concentration, but to what extent is this true and what bias should be expected in human imaging?
PERTINENT FINDINGS: Data obtained as part of a clinical trial showed that PET measurements of radioactivity concentration were approximately 5% higher than an external blood-based reference standard.
IMPLICATIONS FOR PATIENT CARE: These results indicate that modern PET/CT systems are capable of low bias in human (as opposed to phantom) imaging, providing additional support for the use of quantitative PET biomarkers.
Acknowledgments
We thank members of the Radiological Society of North America’s Quantitative Imaging Biomarker Alliance for various helpful discussions.
Footnotes
Published online Oct. 9, 2020
- © 2021 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication June 11, 2020.
- Accepted for publication September 16, 2020.