Abstract
521
Objectives: Combining deep learning-based methods such as convolutional neural networks (CNNs) with the advantage of complementary information from simultaneous positron emission tomography (PET)/ magnetic resonance imaging (MRI), we have previously generated diagnostic quality amyloid PET images with simulated ultra-low (1%) radiotracer dose [1]. Here, we will demonstrate utility of this method with images acquired using actual ultra-low-dose radiotracer injections. Dramatically lowering injected dose will not only reduce radiation risk in subjects but also provide breakthroughs in PET/MRI scanning protocols, allowing for more frequent follow-ups of disease progression.
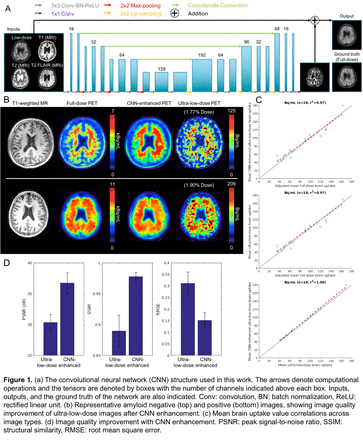
Methods: 50 total subjects were recruited for the study. 32 (19 female, mean±standard deviation [SD]: 68.2±7.1 years) were used for pre-training the network; 328±32 MBq of the amyloid radiotracer 18F-florbetaben were injected into the subject. 18 (8 female, 72.1±8.6 years) were scanned with the ultra-low-dose protocol. These subjects were scanned in two PET/MRI sessions (9 on same day: low-dose session followed by full dose; 9 on separate days: 1- to 42-day interval, mean 19.6 days), with 6.73±3.55 and 302±13 MBq 18F-florbetaben injections respectively (2.3%±1.3% dose for the ultra-low-dose sessions). For all scans, the T1-, T2-, and T2 FLAIR-weighted MR images were acquired simultaneously with PET (90-110 minutes after injection; 83-98 minutes for one subject) on an integrated PET/MR scanner with time-of-flight capabilities. A pre-trained low-dose CNN was trained based on Chen et al. (Figure 1a) [1]. The inputs of the network are the multi-contrast MR images and the simulated low-dose PET image (obtained from 1% random undersampling of the original list-mode data). The network was trained on the full-dose PET image as the ground truth. The last layer of the CNN was fine-tuned using the actual low-dose datasets, with actual low-dose images replacing simulations as inputs. 9-fold cross-validation was used to prevent tuning and testing on the same subjects (16 subjects for training, 2 subjects for testing per network trained). The mean uptake (full-dose images were multiplied by the low-dose percentage) within the brain was analyzed for correlation across image types. For each axial slice, the image quality of the synthesized and low-dose PET images within the brain were compared to the full-dose image using peak signal-to-noise ratio (PSNR), structural similarity (SSIM), and root mean square error (RMSE). Regional (from FreeSurfer) mean standard uptake value ratios (SUVRs, normalized to cerebellar cortex) were compared across image types.
Results: Qualitatively, the synthesized images show marked improvement in noise reduction to the ultra-low-dose image and resemble the ground truth image (Figure 1b). The mean radiotracer uptake within the brain highly correlated across image types, validating the amount of tracer injected for the ultra-low-dose portion (Figure 1c). All three metrics showed dramatic image quality improvement (Figure 1d) from the ultra-low-dose images to the synthesized images. Comparing the regional SUVRs of the synthesized and low-dose images to the full-dose images showed that the mean SUVR differences were close to zero for both image types, but the synthesized images had smaller SDs to their full-dose counterparts (SUVR difference, mean±SD: -0.03±0.16) than the low-dose images (SUVR difference: -0.02±0.17).
Conclusions: This work has shown that accurate amyloid PET images can be generated using trained CNNs with simultaneously-acquired MR images and PET images reconstructed from actual ultra-low-dose radiotracer injections. Acknowledgements: This work was made possible by the Michael J. Fox Foundation, the Stanford Alzheimer's Disease Research Center, the NIH Grant P41-EB015891, GE Healthcare, the Foundation of the ASNR, and Life Molecular Imaging.