Abstract
1035
Introduction: Necroptosis is a complex and regulated caspase-independent cell death mechanism mediated by various protein members, e.g. receptor-interacting protein kinase 1 (RIP1 or RIPK1) involving inflammation. Notably, RIPK1 is up-regulated by microglial cells in Alzheimer`s disease (AD) brains of human and mediates a disease-associated microglial response in AD, which is related to an inflammatory response and a reduction in phagocytic activity. Herein we reported the radiosynthesis and its preliminary evaluation first PET radiotracer of RIPK1/necroptosis.
Methods: Firstly, we designed a strategy to incorporate a carbon-11 radiolabel without altering the original framework of scaffold in the target molecule, CNY-06 (Kd = 3.6 nM), as a promising PET imaging probe for RIP1. Then preliminary in vivo imaging studies were initially performed in wide type (WT) Balb/c mice and the well-established 5XFAD transgenic mice of AD model to study the potential of [11C]CNY-06 to serve as a radiotracer for brain σ1R imaging. WT mice and the 3-4 month female AD model mice were administered [11C]CNY-06 via intravenous bolus injection (100‒150 μCi per animal) and then followed a 60 minutes dynamic PET imaging and a 10 minutes CT scanning. the followed NHPs study was implemented with a male rhesus macaque conducted a 90 minutes dynamic PET/MR scanning after radiotracer administrating. The biodistribution of [11C]CNY-06 in brain was also investigated, the data of different brain regions were acquired in PMOD (PMOD 4.003, PMOD Technologies Ltd., Zurich, Switzerland).
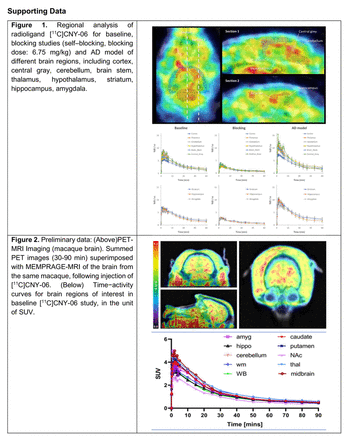
Results: From the analysis of radioactive uptake within brain regions of both WT and AD model mice, [11C]CNY-06 showed high BBB penetration and fast uptake when administered by intravenous bolus injection and exhibited good brain clearance kinetic property during the scanning period. Pretreated unlabeled CNY-06 could significantly decreased the uptake in brain regions, indicated the specific binding and metabolic stability in brain. In AD mice, the radioactivity uptake is 30-40% higher than age and gender matched WT mice across brain regions, which was consistent with the postmortem in vitro results reported (Figure 1). In preliminary NHP study, brain uptake of [11C]CNY-06 was acquired based on PET-MR focused on the head. We identified high brain uptake of 1.0-2.2 in SUV based on PET-MR images. Relatively higher uptake was observed in regions such as midbrain and hypothalamus. (Figure 2).
Conclusions: In summary, the first generation of RIPK1/ necroptosis radiotracer for brain imaging was achieved. In in vivo evaluation, [11C]CNY-06 exhibits great potential in mice and NHP. Using [11C]CNY-06 as a PET probe could great benefit to quantify RIP1 expression in the brain, and it is possible to obtain these information using this new imaging biomarker. Acknowledgements: Support for this work includes a pilot funding from the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital (Changning Wang, USA), National Natural Science Foundation of China (Grant No.81602946, Yu Lan) and Natural Science Foundation of Hubei Province of China (Grant No. 2016CFB258, Yu Lan).