Abstract
1038
Objectives: Defining binding affinity and selectivity for a given target is an important step in developing a new PET radiotracer. This can be particularly challenging when developing radiotracers for protein aggregates found in neurodegenerative disorders. Historically this is done using synthetic aggregates (e.g. heparin-induced tau) or autoradiography on post-mortem tissue slices. However, both approaches have their limitations; synthetic aggregates are not necessarily representative representative of the physiological aggregates, while post-mortem tissues slices often contain small amounts of target protein along with many other components. By way of example, we recently reported specific binding of [18F]FL2-b1 to TDP43 aggregates in Amyotrophic Lateral Sclerosis (ALS) post-mortem human tissue. The study demonstrated colocalization of the tracer with TDP43 aggregates,2 but quantifying Bmax and Kd was challenging because of low TDP43 levels. To confirm specific binding of our tracer to metal-TDP43 aggregates within ALS tissue, and accurately quantify binding affinity, we required a reliable source of TDP43. The objective of this study was to extract (and enrich) TDP43 aggregates from post-mortem human motor cortex from ALS brain samples according using the SarkoSpin protocol.3
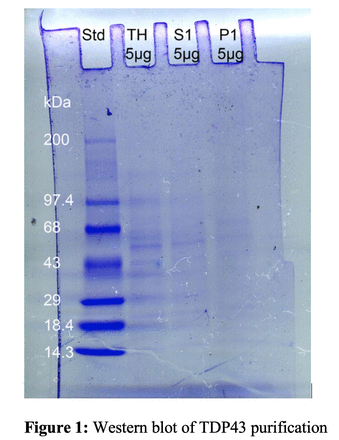
Methods: Frozen tissue from motor or frontal cortex sections of ALS subjects was homogenized and solubilized in order to precipitate phosphorylated TDP43 (pTDP43) using the SarkoSpin protocol published by Laferrière (combining a harsh solubilization with nuclease treatment and a single centrifugation step).3 Initial evaluation of the protein concentration was tested by Bradford’s assay. To determine separation of pTDP43 aggregates from its normal counterpart, western blotting was utilized with 5 µg protein per sample and visualized by Coomassie brilliant blue.Results: Visualization of the protein contents of the tissue homogenate (TH), supernatant (S1), and pellet (P1) by western blot revealed that normal TDP43 remained in the supernatant, completely removed from the insoluble protein pellet (see Figure 1). This confirmed the presence of a band at 43 kDa in both the tissue homogenate and supernatant, which was not present in the pellet (P1). Conclusions: Removal of TDP43 from the insoluble protein aggregate pellet obtained from post mortem ALS tissue has been confirmed using Western blotting techniques. Quantification of the aggregate concentration using an ELISA assay is ongoing and will also be reported. The enrichment of pTDP43 aggregates will facilitate quantitative binding studies with new TDP43 radiotracers being developed in our laboratory to ensure specific binding to the diseased protein before advancing to preclinical (and clinical) imaging studies. References [1] Choi, J.-S.; Braymer, J. J.; Lim, M. H.; et al. Proc. Natl. Acad. Sci. 2010, 107 (51), 21990-21995. [2]Tanzey, S.S.; Brooks, A.F.; Shao, X.; Desmond, T.; Scott, P.J.H. J Cereb Blood Flow Metab. 2019, 39 suppl: 524-608.2. [3] Laferriere, F.; Maniecka, Z., Perez-Berlanga, M. et al. Nature Neuroscience. 2019, 22, 65-77.