Abstract
596
Objectives: We compared the clinical value of 18F-FES PET/CT and 18F-FDG PET/CT and investigated whether and how 18F-FES PET/CT affects the implemented management of newly diagnosed oestrogen receptor positive breast cancer patients.
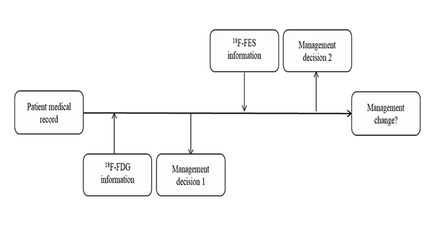
Methods: We retrospectively analysed nineteen female patients newly diagnosed with immunohistochemistry (IHC) confirmed ER positive breast cancer who underwent 18F-FES and 18F-FDG PET/CT within 1 week in our centre. The sensitivity of 18F-FES and 18F-FDG in diagnosed lesions were compared using the McNemar test. To investigate the definite clinical impact of 18F-FES on managing patients with newly diagnosed ER positive breast cancer, we designed two kinds of questionnaires. Referring physicians completed the first questionnaire based on the 18F-FDG report to propose the treatment regime, and the second was completed immediately after reviewing the imaging report of 18F-FES to indicate intended management changes.
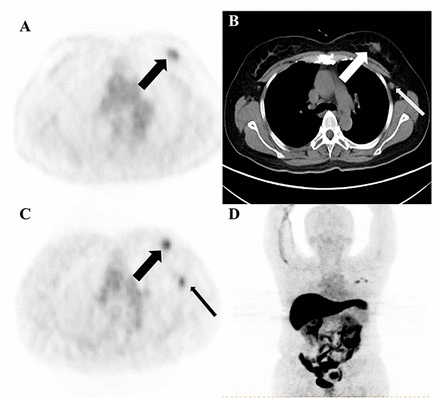
Results: In total, 238 lesions were analysed in 19 patients with newly diagnosed ER positive breast cancer. Lesion detection was achieved in 216 sites with 18F-FES PET and in 197 sites with 18F-FDG PET/CT. These results corresponded to sensitivities of 90.8% for 18F-FES versus 82.8% for 18F-FDG PET/CT in diagnosed lesions. Thirty-five physicians were given the questionnaires referring to the treatment strategy, with 27 of them completing both questionnaires. The application of 18F-FES in addition to 18F-FDG PET/CT changed the management in 26.3% of the 19 patients with newly diagnosed ER positive breast cancer.
Conclusions: Performing 18F-FES PET/CT in newly diagnosed ER positive breast cancer patients increases the value of diagnosis equivocal lesions and treatment management compared with 18F‑FDG PET/CT.
Sensitivity comparison of 18F-FDG and 18F-FES for suspected disease lesions
Sensitivity comparison of 18F-FDG and 18F-FES for only suspected without liver lesions
The proportion of changes in management after 18F‑FES PET.