Abstract
A previous study from this laboratory suggested that 11C-yohimbine, a selective α2-adrenoceptor antagonist, is an appropriate ligand for PET of α2 adrenoceptors that passes readily from blood to brain tissue in pigs but not in rodents. To test usefulness in humans, we determined blood–brain clearances, volumes of distribution, and receptor availability by means of PET with 11C-yohimbine in healthy male adults. Methods: We recorded the distribution of 11C-yohimbine with 90-min dynamic PET and sampled arterial blood to measure intact 11C-yohimbine in plasma. For analysis, we coregistered PET images to individual MR images and automatically identified 27 volumes of interest. We used 1-tissue-compartment graphical analysis with 6 linearized solutions of the fundamental binding equation, with the metabolite-corrected arterial plasma curves as input function, to estimate the kinetic parameters of 11C-yohimbine. With the lowest steady-state distribution volume (VT), determined in the corpus callosum, we calculated the binding potential (receptor availability) of the radioligand in other regions. Results: The linear regressions yielded similar estimates of the kinetic parameters. The cortical values of VT ranged from 0.82 mL cm−3 in the right frontal cortex to 0.46 mL cm−3 in the corpus callosum, with intermediate VT values in subcortical structures. Binding potentials averaged 0.6–0.8 in the cortex and 0.2–0.5 in subcortical regions. Conclusion: The maps of 11C-yohimbine binding to α2 adrenoceptors in human brain had the highest values in cortical areas and hippocampus, with moderate values in subcortical structures, as found also in vitro. The results confirm the usefulness of the tracer 11C-yohimbine for mapping α2 adrenoceptors in human brain in vivo.
Among the α2 adrenoceptors, the autoreceptors are located presynaptically on noradrenergic neurons, where they regulate firing of noradrenergic neurons as well as noradrenaline synthesis and release (1). Three α2-adrenoceptor subtypes have been identified, including the cloned human α2 adrenoceptors α2-C10, α2-C2, and α2-C4, labeled according to the chromosomal localization of the genes (2–4). The three α2-adrenoceptor subtypes are known as α2a, α2b, and α2c in the pharmacologic nomenclature (5).
Dense expression of α2 adrenoceptors occurs in the cerebral cortex, hippocampal formation, hypothalamus, and locus coeruleus, with intermediate-to-moderate binding in subcortical structures and low expression in white matter (6,7). Degeneration of the noradrenergic neurotransmitter system and specific loss of α2 adrenoceptors is implicated in neurodegenerative disorders (8). Imaging of α2 adrenoceptors therefore is essential to understanding the role of the noradrenergic system in these disorders. However, no PET radioligands selective for α2 adrenoceptors have passed successfully from preclinical evaluation to clinical usefulness (9,10).
We recently developed 11C-yohimbine for use with PET. Yohimbine binds to all α2-adrenoceptor subtypes and has moderate affinity for the 5HT1A receptors as well (11). In porcine brain, 11C-yohimbine enters brain tissue readily and binds specifically in brain areas with known high densities of α2 adrenoceptors. In rat brain, 11C-yohimbine is excluded from entry into brain tissue by the action of permeability glycoprotein (12). In porcine brain, both unlabeled yohimbine and RX821002 (2-(2-methoxy-1,4-benzodioxan-2yl)-2-imidazoline), a highly selective α2-adrenoceptor antagonist, displace 11C-yohimbine binding (11). These results prompted us to test 11C-yohimbine as a radioligand for use in humans, particularly with respect to the ability of the tracer to readily cross the blood–brain barrier.
MATERIALS AND METHODS
Subjects
The Committee on Ethics of the Central Denmark Region approved this study, and all subjects provided written informed consent before participation in this study. None of the subjects had any neurologic or psychiatric disorder. We recruited 6 young male subjects with a mean age of 28.7 y (SEM, ±4.1 y) and an age range of 24–35 y to participate in the study. Subject 6 was excluded from analysis after the PET session, as this subject received a dose of radioactivity almost one order of magnitude lower than the other subjects and showed the highest rate of metabolism of yohimbine in the circulation (corresponding to the CYP2D6 status given in Table 1), resulting in the almost complete disappearance of radioactivity from the circulation.
Injected Radioactivity Dose, Yohimbine Mass, and CYP2D6 Status
Radiochemistry, Metabolites, and CYP2D6 Status
The synthesis of 11C-yohimbine has been described in detail elsewhere (11). Briefly, cyclotron-produced 11C-carbon dioxide was converted to 11C-methyliodide and trapped in dimethylsulfoxide (300 μL) containing NaOH (1 μL, 3 M) and yohimbinic acid (1 mg) in a 1-mL vial. This mixture was heated at 80°C for 3 min. 11C-yohimbine was purified by semipreparative high-performance liquid chromatography. The mobile phase, consisting of 75% aqueous 70 mM Na2HPO4 and 25% ethanol, was delivered at a rate of 5 mL/min to a LUNA C18(2) 250 × 10 mm semipreparative column (Phenomenex) with online radio wave and ultraviolet–visible (280 nm) detection. The fraction containing 11C-yohimbine (retention time, 7–8 min) was collected and diluted with 5 mL of sterile saline and filtered through a sterile 0.22-μm filter to obtain a total of 10 mL of product solution. This procedure gave 1–2 GBq of 11C-yohimbine with radiochemical purity greater than 99% in a sterile formulation ready for injection. With regard to metabolites of 11C-yohimbine in circulation, the fraction of untransformed 11C-yohimbine was measured by radio–high-performance liquid chromatography in extracts of plasma from samples taken at 2, 5, 10, 20, 40, 60, and 90 min after injection. We also genotyped the cytochrome P450 (CYP) system with regard to the CYP2D6 isoform at the laboratory Filedelfia, Denmark.
Yohimbinic acid monohydrate, yohimbine hydrochloride, dimethylsulfoxide, and acetonitrile were purchased from Sigma Aldrich.
Image Acquisition, Registration, and Segmentation
Subjects reclined in the scanner, an ECAT High Resolution Research Tomograph (HRRT; CTI/Siemens), and the head was positioned and immobilized in a custom-built head holder. A 6-min transmission scan was obtained, and a 90-min dynamic PET scan consisting of 28 frames increasing in duration from 15 s in the beginning to 10 min at the end of the scan (8 × 15 s, 4 × 30 s, 6 × 60 s, 4 × 300 s, 6 × 600 s) was recorded in 3-dimensional mode on administration of 11C-yohimbine. An ordered-subsets expectation maximization 3-dimensional ordinary Poisson algorithm including point spread function modeling, with 10 iterations and 16 subsets, was used for reconstruction, resulting in 256 × 256 × 207 images at a resolution of 1.8 mm in full width at half maximum. Blood samples were collected at 5-s intervals in the beginning increasing to intervals of 10 min at the end of the scan. The subjects underwent T1-weighted MR imaging. We used PMOD software, version 3.5, and the module PNEURO (PMOD Technologies Ltd.) for model-based image coregistration and segmentation of volumes of interest (VOIs). First, we averaged the dynamic images from frames 5–25 to provide a sufficient anatomic PET image. We then coregistered the anatomic PET images to the individual anatomic MR images by a rigid coregistration method using a mutual-information algorithm. Individual MRI scans were spatially normalized to the Montreal Neurological Institute T1 template, and the transformation matrices were used to bring the dynamic PET scan into Montreal Neurological Institute space. VOIs were automatically outlined on the normalized MR images according to the Hammers maximum probability atlas, which is implemented in PMOD (13). The VOIs were intersected with a 50% gray matter probability mask and then applied to the spatially normalized dynamic PET scans to obtain time–activity curves for 27 selected VOIs (Table 2).
Kinetic Parameters of 11C-Yohimbine
Kinetic Analysis of 11C-Yohimbine Uptake and Distribution
The kinetic parameters of 11C-yohimbine in the human brain were estimated with nonlinear 1- and 2-tissue-compartment models. There were no significant differences between estimates of 11C-yohimbine volumes of distribution or Akaike information criterion scores between these models. However, the nonlinear 2-tissue-compartment model produced nonphysiologic estimates of the kinetic parameters in some instances. For simplicity of analysis and illustration, we chose to compare the results of 6 linearizations of the 1-tissue-compartment model, as presented in the paragraphs below.
The distribution of a tracer as a function of time is governed by linear differential equations with constant transfer coefficients. In the case of a tracer that enters a brain tissue compartment in which bound and unbound tracer are in steady state, the distribution of the tracer is governed by the exchange between the circulation and the tissue compartment across the blood–brain and other potential barriers: Eq. 1where
Eq. 1where  (kBq/mL) is the tracer quantity in the vascular compartment with the volume
(kBq/mL) is the tracer quantity in the vascular compartment with the volume 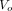 (mL/cm3) and the concentration
(mL/cm3) and the concentration  (kBq/mL) as function of time, and
(kBq/mL) as function of time, and Eq. 2where
Eq. 2where  (kBq/mL) is the quantity of tracer in the tissue compartment,
(kBq/mL) is the quantity of tracer in the tissue compartment,  (mL/cm3/min) is the unidirectional clearance of tracer from the circulation, and
(mL/cm3/min) is the unidirectional clearance of tracer from the circulation, and  (min−1) is the rate constant of tracer efflux from the tissue compartment, equal to the ratio of the unidirectional clearance
(min−1) is the rate constant of tracer efflux from the tissue compartment, equal to the ratio of the unidirectional clearance  and the total steady-state volume of distribution
and the total steady-state volume of distribution 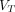 (mL/cm3), here defined as
(mL/cm3), here defined as Eq. 3where
Eq. 3where  (min−1) is the rate constant of efflux of dissolved (unbound) tracer from the tissue compartment, where the physical volume of distribution of tracer in solution is the volume of nondisplaceable tracer
(min−1) is the rate constant of efflux of dissolved (unbound) tracer from the tissue compartment, where the physical volume of distribution of tracer in solution is the volume of nondisplaceable tracer  (mL/cm3) (from which it cannot be displaced by blockade of the binding) and the binding potential, or steady-state ratio between bound and unbound tracer, is
(mL/cm3) (from which it cannot be displaced by blockade of the binding) and the binding potential, or steady-state ratio between bound and unbound tracer, is  .
.
When solved, the 2 differential equations yield Eq. 4and
Eq. 4and Eq. 5from which the unknown quantity
Eq. 5from which the unknown quantity  can be eliminated by substitution of
can be eliminated by substitution of  ,
, Eq. 6which can be further reduced when the
Eq. 6which can be further reduced when the  ratio approaches zero,
ratio approaches zero, Eq. 7at a time that can be judged from the ratio as a function of time,
Eq. 7at a time that can be judged from the ratio as a function of time,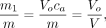 Eq. 8where
Eq. 8where  is an apparent volume of distribution (mL/cm3) that equals the ratio of the total quantity of tracer in the tissue and the arterial concentration of the tracer as a function of time. With a vascular volume close to 3% in brain tissue, the volume
is an apparent volume of distribution (mL/cm3) that equals the ratio of the total quantity of tracer in the tissue and the arterial concentration of the tracer as a function of time. With a vascular volume close to 3% in brain tissue, the volume 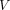 , equal to the ratio
, equal to the ratio  , cannot exceed more than 3 mL cm−3 for the vascular tracer in brain to be less than 1% of the total radioactivity in the brain. After that time, the transfer of 11C-yohimbine to the tissue from the vascular compartment can be described with a single-compartment equation that replaces the 2-compartment equation for times great enough to render the radioactivity in the vascular volume negligible in relation to the total radioactivity in the tissue, which then obeys the equation,
, cannot exceed more than 3 mL cm−3 for the vascular tracer in brain to be less than 1% of the total radioactivity in the brain. After that time, the transfer of 11C-yohimbine to the tissue from the vascular compartment can be described with a single-compartment equation that replaces the 2-compartment equation for times great enough to render the radioactivity in the vascular volume negligible in relation to the total radioactivity in the tissue, which then obeys the equation, Eq. 9Rearrangement of this basic equation yields linear plots with positive or negative slopes. Here, we compare 6 different linear plots that estimate the kinetic parameters that govern uptake and distribution of 11C-yohimbine in the brain (14–16).
Eq. 9Rearrangement of this basic equation yields linear plots with positive or negative slopes. Here, we compare 6 different linear plots that estimate the kinetic parameters that govern uptake and distribution of 11C-yohimbine in the brain (14–16).
The kinetic variables calculated from the radioactivity records include the apparent volume of distribution as a function of the duration of circulation of the tracer (in units of mL cm−3), Eq. 10as well as the apparent net clearance of the tracer from plasma to brain as a function of circulation time (in units of mL/cm3/min),
Eq. 10as well as the apparent net clearance of the tracer from plasma to brain as a function of circulation time (in units of mL/cm3/min), Eq. 11and the apparent residence time in the tissue (in units of minutes),
Eq. 11and the apparent residence time in the tissue (in units of minutes), Eq. 12such that
Eq. 12such that  . When the primary variables are combined, they define 2 plots of negative slope (N1 and N2), or 4 plots of positive slope (P1–P4). In the “clearance plot” (N1), the ordinate intercept is the apparent clearance,
. When the primary variables are combined, they define 2 plots of negative slope (N1 and N2), or 4 plots of positive slope (P1–P4). In the “clearance plot” (N1), the ordinate intercept is the apparent clearance, 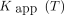 , of tracer from plasma to brain, and the slope is −
, of tracer from plasma to brain, and the slope is − such that the abscissa intercept is the estimate of
such that the abscissa intercept is the estimate of 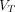 (17,18),
(17,18), Eq. 13whereas in the reverse “reciprocal ratio plot” (N2) (19),
Eq. 13whereas in the reverse “reciprocal ratio plot” (N2) (19),  is plotted against
is plotted against  , such that the ordinate intercept is the estimate of the VT,
, such that the ordinate intercept is the estimate of the VT, Eq. 14The first of the positive slope plots is the “ratio plot” (P1) (17), where the inverse of the unidirectional clearance
Eq. 14The first of the positive slope plots is the “ratio plot” (P1) (17), where the inverse of the unidirectional clearance  is the ordinate intercept and the slope is the inverse of
is the ordinate intercept and the slope is the inverse of  ,
, Eq. 15such that the second of the positive slope plots, the Logan plot (P2), is obtained by reversing the equation, as originally published by Logan et al. (20),
Eq. 15such that the second of the positive slope plots, the Logan plot (P2), is obtained by reversing the equation, as originally published by Logan et al. (20),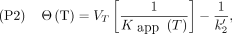 Eq. 16where
Eq. 16where 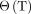 is plotted against the inverse of clearance, such that the positive slope represents
is plotted against the inverse of clearance, such that the positive slope represents 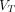 . The plot becomes linear when the system has reached steady state.
. The plot becomes linear when the system has reached steady state.
The third positive slope, or “reciprocal time,” plot originally was derived by Reith et al. (21), who plotted the reciprocal of  against the reciprocal of
against the reciprocal of  ,
, Eq. 17where the steady-state volume is obtained from the slope and the ordinate intercept. In the last of the 4 positive slope plots, the axes of P3 have been reversed,
Eq. 17where the steady-state volume is obtained from the slope and the ordinate intercept. In the last of the 4 positive slope plots, the axes of P3 have been reversed, Eq. 18where
Eq. 18where  is the reciprocal of the ordinate intercept. We calculated BPND as the ratio of the volume
is the reciprocal of the ordinate intercept. We calculated BPND as the ratio of the volume  in ROIs with specifically displaceable binding and the volume
in ROIs with specifically displaceable binding and the volume  of distribution in a chosen reference region with specifically nondisplaceable binding, here selected as the volume determined for the corpus callosum (
of distribution in a chosen reference region with specifically nondisplaceable binding, here selected as the volume determined for the corpus callosum ( ) (22).
) (22). Eq. 19
Eq. 19
Data Analysis
Analyses were performed in GraphPad Prism 6.0 and results are reported as mean (±SEM). The correlation of estimates of  between the different linear models was tested by linear regression of frontal cortex
between the different linear models was tested by linear regression of frontal cortex  determined with P4 versus the other models.
determined with P4 versus the other models.
RESULTS
Brain Time–Activity Curves and Metabolites
The tracer readily entered brain tissue, as revealed by the standardized uptake value curves and the estimates of the unidirectional blood–brain clearances ( ) of the tracer. Standardized uptake value curves for all 27 VOIs of one subject are shown in Figure 1A. We used the fraction of radioactivity in plasma, relative to the radioactivity in a given VOI, as shown in Figure 1B, to determine the time after which the fraction would be negligible and the plots henceforth linear, exemplified by frontal cortex and hippocampus, where, on average, the vascular tracer amounted to less than 6% after 12.5 min. The fraction of intact tracer declined in plasma as a function of time. When we genotyped the CYP mixed-function oxidase CYP2D6 in all subjects, we classified 2 subjects as fast metabolizers and 3 subjects as moderate metabolizers. We classified one subject with no functional CYP2D6 genetic variants as a nonmetabolizer as shown in Table 1 and Figure 2.
) of the tracer. Standardized uptake value curves for all 27 VOIs of one subject are shown in Figure 1A. We used the fraction of radioactivity in plasma, relative to the radioactivity in a given VOI, as shown in Figure 1B, to determine the time after which the fraction would be negligible and the plots henceforth linear, exemplified by frontal cortex and hippocampus, where, on average, the vascular tracer amounted to less than 6% after 12.5 min. The fraction of intact tracer declined in plasma as a function of time. When we genotyped the CYP mixed-function oxidase CYP2D6 in all subjects, we classified 2 subjects as fast metabolizers and 3 subjects as moderate metabolizers. We classified one subject with no functional CYP2D6 genetic variants as a nonmetabolizer as shown in Table 1 and Figure 2.
(A) Examples of standardized uptake value curves for all 27 VOIs of 11C-yohimbine presented for subject 5 (abscissa is mid-frame time [minutes] and ordinate is standardized uptake value of radioactivity in VOIs). Blue line shows corpus callosum, which was used as reference region for calculation of binding potentials (BPND). (B) Radioactivity in plasma, expressed as fraction of radioactivity in VOIs, is shown on ordinate, and abscissa represents time (minutes).
Fractions of unchanged 11C-yohimbine were interpolated to match time points of tissue time–activity curves, presented here for all 6 subjects. Ordinate represents intact 11C-yohimbine, and abscissa shows time (minutes).
Blood–Brain Clearances, Efflux Rates, Volumes of Distribution, and Binding Potentials of 11C-Yohimbine
After the time of negligible tracer in the vascular space (12.5 min), the N1–P4 plots based on unmetabolized tracer in plasma assumed linearity and yielded comparable estimates of the 3 parameters 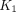 ,
,  , and
, and  as shown in Figure 3. In line with this observation, we noted a highly significant correlation between estimates of
as shown in Figure 3. In line with this observation, we noted a highly significant correlation between estimates of  in the frontal cortex determined with the plot P4 method and the remaining plots with P < 0.0001 and mean R2 = 0.9974 (±0.0007). As the estimates were comparable, only
in the frontal cortex determined with the plot P4 method and the remaining plots with P < 0.0001 and mean R2 = 0.9974 (±0.0007). As the estimates were comparable, only  and
and  values from plot P4 and the efflux rate constant
values from plot P4 and the efflux rate constant  from plot N2 are presented here. We calculated the
from plot N2 are presented here. We calculated the  values listed in Table 2 for selected VOIs from the ratios of
values listed in Table 2 for selected VOIs from the ratios of  estimates to the estimate in the corpus callosum. The mean values of
estimates to the estimate in the corpus callosum. The mean values of  ranged from 0.82 (±0.16) mL cm−3 in the right frontal cortex to 0.46 (±0.09) mL cm−3 in the corpus callosum, as also listed in Table 2. Estimates of
ranged from 0.82 (±0.16) mL cm−3 in the right frontal cortex to 0.46 (±0.09) mL cm−3 in the corpus callosum, as also listed in Table 2. Estimates of  and
and 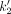 are listed in Table 2 for selected VOIs.
are listed in Table 2 for selected VOIs.
Six linearized solutions were used to derive linear regression estimates of kinetic parameters, using metabolite-corrected plasma curves as input function: plot N1 (A), plot N2 (B), plot P1 (C), plot P2 (D), plot P3 (E), plot P4 (F). Blue lines represent corpus callosum.
The estimates of  and
and  ranged from 0.020 (±0.003) mL cm−3 min−1 and 0.023 (±0.001) min−1, respectively, in the right frontal cortex, to 0.009 (±0.001) mL cm−3 min−1 and 0.019 (±0.003) min−1, respectively, in the corpus callosum. The estimates of
ranged from 0.020 (±0.003) mL cm−3 min−1 and 0.023 (±0.001) min−1, respectively, in the right frontal cortex, to 0.009 (±0.001) mL cm−3 min−1 and 0.019 (±0.003) min−1, respectively, in the corpus callosum. The estimates of 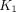 are consistent with blood–brain extraction fractions of 5%. A parametric image of the average estimates of
are consistent with blood–brain extraction fractions of 5%. A parametric image of the average estimates of  in 5 subjects obtained with the plot P2 (Logan plot) is shown in Figure 4. In subject 6, the parametric image was of unacceptably poor quality because of a combination of minimum dose of radioactivity and maximum rate of metabolism in plasma. Mean values of
in 5 subjects obtained with the plot P2 (Logan plot) is shown in Figure 4. In subject 6, the parametric image was of unacceptably poor quality because of a combination of minimum dose of radioactivity and maximum rate of metabolism in plasma. Mean values of  , calculated and averaged from estimates of
, calculated and averaged from estimates of  obtained from regions including the corpus callosum as reference by means of plot P4, are listed in Table 2, where they range from 0.6 to 0.8 in the cortex and from 0.2 to 0.5 in subcortical regions.
obtained from regions including the corpus callosum as reference by means of plot P4, are listed in Table 2, where they range from 0.6 to 0.8 in the cortex and from 0.2 to 0.5 in subcortical regions.
Average parametric images of voxelwise  estimates in 5 of 6 subjects, estimated with P2 plot (Logan plot). Color bar gives estimates of
estimates in 5 of 6 subjects, estimated with P2 plot (Logan plot). Color bar gives estimates of  in units of mL cm−3.
in units of mL cm−3.
DISCUSSION
Animal studies from this laboratory have suggested that 11C-yohimbine is a selective and displaceable α2-adrenoceptor antagonist when used in tracer concentrations (11,12). To measure accumulation of the tracer in human brain, we determined the uptake and binding of 11C-yohimbine in 5 healthy male volunteers (subject 6 was excluded). The results revealed about 5% extraction of the tracer, sufficient for determination of regionally averaged uptake in 5 volunteers, and similarly acceptable for voxel-based mapping in only 5 of the 6 volunteers.
Linearized solutions of the underlying differential equations such as the Logan plot (P2) were used here to graphically estimate the kinetic parameters of 11C-yohimbine (17–21). We analyzed the present data with 6 different linearized solutions, including the Logan plot, to identify the plot most suitable for future applications of 11C-yohimbine. All solutions, here abbreviated N1–P4, of which P2 is the Logan plot, adequately fitted the data after the plots assumed linearity approximately 12.5 min after administration of the bolus of radioligand as shown in Figure 3. The estimates of 11C-yohimbine  in the frontal cortex determined with plot P4 significantly correlated with the 5 other linearizations (P < 0.0001). The estimates ranged from 0.82 mL cm−3 in the right frontal cortex to 0.72 mL cm−3 in the left insula and 0.77 mL cm−3 in the left hippocampus. Subcortical structures, including the thalamus, caudate nucleus, putamen, and amygdala, had lower estimates of
in the frontal cortex determined with plot P4 significantly correlated with the 5 other linearizations (P < 0.0001). The estimates ranged from 0.82 mL cm−3 in the right frontal cortex to 0.72 mL cm−3 in the left insula and 0.77 mL cm−3 in the left hippocampus. Subcortical structures, including the thalamus, caudate nucleus, putamen, and amygdala, had lower estimates of  . Surprisingly, white matter, excluding the corpus callosum, also had a moderate volume of distribution of 11C-yohimbine (0.59 mL cm−3), which may reflect the influence of partial volume.
. Surprisingly, white matter, excluding the corpus callosum, also had a moderate volume of distribution of 11C-yohimbine (0.59 mL cm−3), which may reflect the influence of partial volume.
Obtained from the linearized plots, the estimates of unidirectional blood–brain clearance of 11C-yohimbine,  , had a 2-fold range whereas the rate of washout of the tracer had a narrow range from the right frontal cortex to the corpus callosum (Table 2). In the study by Hume et al. (23), the
, had a 2-fold range whereas the rate of washout of the tracer had a narrow range from the right frontal cortex to the corpus callosum (Table 2). In the study by Hume et al. (23), the 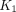 value in the frontal cortex of another PET ligand for α2 adrenoceptors, 11C-RS-15385-197, was 0.009 mL/cm3/min, which is close to
value in the frontal cortex of another PET ligand for α2 adrenoceptors, 11C-RS-15385-197, was 0.009 mL/cm3/min, which is close to  in the corpus callosum in the current study. In contrast, the tracer 11C-mirtazapine had a
in the corpus callosum in the current study. In contrast, the tracer 11C-mirtazapine had a  value approximately 10 times higher than that for 11C-yohimbine. However, 11C-mirtazapine lacks selectivity for α2 adrenoceptors (9).
value approximately 10 times higher than that for 11C-yohimbine. However, 11C-mirtazapine lacks selectivity for α2 adrenoceptors (9).
Although the distribution volumes of 11C-yohimbine are modest compared with other PET radioligands and with 11C-mirtazapine, its binding potential is comparable to other noradrenergic tracers in successful use. For example, the binding potential of (S,S)-11C-methylreboxetine, an antagonist of noradrenaline transporters, is 0.52 in the thalamus, which receives noradrenergic innervation (24).
Here, we calculated binding potentials as estimates of receptor availability as the ratio of  estimated in VOIs with assumed displaceable binding, compared with a chosen reference region, corpus callosum. Hume et al. (23) used the thalamus as a reference region based on the assumption that it contains few α2-adrenoceptor binding sites. However, some thalamic nuclei show dense expression of α2 adrenoceptors, which may complicate the choice of thalamus as a reference region (6). In vitro studies have shown that white matter and the corpus callosum have negligible α2-adrenoceptor binding sites and thus may be considered for use as a reference region. The tentative choice of the corpus callosum as a reference region here was based on the finding of the lowest
estimated in VOIs with assumed displaceable binding, compared with a chosen reference region, corpus callosum. Hume et al. (23) used the thalamus as a reference region based on the assumption that it contains few α2-adrenoceptor binding sites. However, some thalamic nuclei show dense expression of α2 adrenoceptors, which may complicate the choice of thalamus as a reference region (6). In vitro studies have shown that white matter and the corpus callosum have negligible α2-adrenoceptor binding sites and thus may be considered for use as a reference region. The tentative choice of the corpus callosum as a reference region here was based on the finding of the lowest  , as estimated with the plot P4. The high selectivity of the PET ligand, 11C-yohimbine, for α2-adrenoceptors (11), with the highest
, as estimated with the plot P4. The high selectivity of the PET ligand, 11C-yohimbine, for α2-adrenoceptors (11), with the highest  values being in regions with known high density of α2 adrenoceptors, indicates that this ligand is useful for in vivo imaging of α2 adrenoceptors in humans.
values being in regions with known high density of α2 adrenoceptors, indicates that this ligand is useful for in vivo imaging of α2 adrenoceptors in humans.
The CYP-450 system and specifically the CYP2D6 enzyme in the liver are involved in the metabolism of yohimbine in the circulation, yielding 2 metabolites, 10-OH-yohimbine and 11-OH-yohimbine (25). We genotyped the CYP2D6 enzyme and classified subjects as fast, moderate, or low metabolizers, depending on the number of functional CYP2D6 variants, with one subject classified as a nonmetabolizer. As expected, the fraction of unchanged 11C-yohimbine remained close to unity in this subject, whereas moderate and fast metabolizers had substantial breakdown of 11C-yohimbine. The results are consistent with an earlier study of yohimbine metabolism (25), which also identified one subject as a nonmetabolizer. Yohimbine metabolites may have some action at α2 adrenoceptors, albeit with lower affinity when compared with native yohimbine (26). Labeled 11-OH-yohimbine could hypothetically contribute to radioactivity in the brain, as samples of cerebrospinal fluid in previous studies have revealed transport across the blood–brain barrier (27). We plan to study radioactively labeled metabolites of yohimbine to estimate the degree to which they cross the blood–brain barrier and bind to targets in the brain.
The α2 adrenoceptors play important roles in the regulation of noradrenergic neurotransmission and are implicated in the pathology of neurodegenerative and neuropsychiatric disorders (27). It is likely, therefore, that in vivo imaging of α2 adrenoceptors with 11C-yohimbine will provide insights into the underlying mechanisms of these disorders.
CONCLUSION
Here, we present 11C-yohimbine as a PET radioligand for vivo imaging of α2 adrenoceptors in humans. The relative distribution of 11C-yohimbine is similar to that predicted from in vitro studies, with the highest binding being in the cortex and hippocampus, and the binding suggests that 11C-yohimbine may be a suitable ligand for future studies of the role of α2 adrenoceptors in neurodegenerative and neuropsychiatric disorders of the human brain.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This project received financial support from the Lundbeck Foundation (grant 6970) and from the Danish Council for Independent Research, Medical Sciences (grant 0602-02700). No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jan. 29, 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication August 10, 2014.
- Accepted for publication November 18, 2014.











