Abstract
The aim of this study was to investigate the value of standardized uptake values (SUVs) and metabolic tumor volume (MTV) in 18F-FDG PET/CT to predict the survival of patients with locally advanced non–small cell lung cancer during the early stage of concurrent chemoradiotherapy. Methods: A total of 53 patients were included in the prospective study. All patients were evaluated by 18F-FDG PET before and after 40 Gy of radiotherapy with a concurrent cisplatin-based chemotherapy regimen. Semiquantitative assessment was used to determine the maximum and mean SUVs (SUVmax and SUVmean, respectively) and MTV of the primary tumor. The cutoffs for changes in SUVmax, SUVmean, and MTV (37.2%, 41.7%, and 29.7%, respectively) determined in a previous study were used with Kaplan–Meier curves to separate the groups. The prognostic significance of PET/CT parameters and other clinical variables was assessed using Cox regression analysis. Results: Overall survival (OS) at 1 and 2 y was 83.0% (46/53) and 52.8% (28/53), respectively. Survival curves for SUVmean and MTV were significantly different using the cutoffs. However, Cox regression analysis showed that the only prognostic factor for OS was a decrease in MTV. Conclusion: The use of repeated 18F-FDG PET to assess survival early during concurrent chemoradiotherapy is possible in patients with locally advanced non–small cell lung cancer. A decrease in MTV according to 18F-FDG uptake by the primary tumor correlates with higher long-term OS.
Lung cancer is the leading cause of cancer death in the world, and 80%–85% of lung cancer cases are classified as non–small cell lung cancer (NSCLC) (1). The combination of platinum-based chemotherapy and radiotherapy is a commonly recommended standard curative approach in unresectable locally advanced disease (2). A need arises to predict therapy response and survival at an early phase on an individual-patient basis, possibly leading to improved tumor control, a reduction in side effects, and eventually avoidance of the futile costs of ineffective treatment. The TNM staging system is considered the most important tool to estimate prognosis and to date is the most important guide in treatment decisions (3). However, the TNM staging system provides an incomplete biologic profile of NSCLC, does not always provide a satisfactory explanation for differences in recurrence and survival, and is therefore far from perfect as a prognostic indicator (4).
In recent years, PET imaging using the tracer 18F-FDG has incorporated metabolic tumor function with anatomic localization when integrated with CT imaging. One of the great advantages of 18F-FDG PET is that it not only can observe but also can quantify 18F-FDG uptake to distinguish metabolically highly active from less active tumor tissues and therefore offers an opportunity for noninvasive, in vivo tissue characterization. Hence, the number of clinical applications for 18F-FDG PET/CT in NSCLC continues to increase. 18F-FDG PET imaging is routinely performed for staging, restaging, treatment planning, and follow-up. Studies have shown that the degree of 18F-FDG uptake by the tumor, as assessed with maximum standardized uptake value (SUVmax), is a significant prognostic factor in NSCLC (5–8). More recently, metabolic tumor volume (MTV) has been explored as a measure of metabolic tumor burden. MTV indicates the volume of metabolically active tumor, typically assessed with semiautomatic PET analysis software.
Our previous study showed that changes in SUV and MTV between 2 serial 18F-FDG PET/CT scans, before and after initial concurrent chemoradiotherapy (CCRT), allow prediction of treatment response in locally advanced NSCLC (9). In the present study, long-term follow-up of the previous study investigated the correlation between SUV and MTV changes in the primary tumor on repeated 18F-FDG PET/CT and overall survival (OS).
MATERIALS AND METHODS
Materials
This study included 63 patients with advanced NSCLC diagnosed by histologic or cytologic examination as tumor stage IIIA or IIIB. All patients had a measurable primary tumor according to the Response Evaluation Criteria in Solid Tumors, and all had undergone radiotherapy with concurrent chemotherapy. The patients were selected consecutively from those treated at the Department of Radiation Oncology in Shandong Cancer Hospital between September 2008 and June 2011. The study was approved by the institutional review board of Shandong Cancer Hospital, and all subjects signed an informed consent form. Initial routine staging procedures consisted of a clinical examination including lung function tests, bronchoscopy, and mediastinoscopy; contrast-enhanced helical CT of the chest and abdomen; and CT or MR imaging of the brain with and without contrast material. Patients were excluded if they were diabetic or had undergone surgery, previous chemotherapy, or radiotherapy. Two PET/CT scans were included as a component of the initial staging or during the course of therapy. Clinicopathologic data are shown in Table 1.
Clinicopathologic Features of 53 Patients with NSCLC
CCRT
All patients underwent radiation therapy, which was delivered with megavoltage equipment (6 MV). The delivery technique was intensity-modulated radiation therapy/3-dimensional conformal radiation therapy. Forty grays of irradiation were given conventionally fractionated at five 2-Gy doses per week, and irradiation was accelerated later in the course, with a hyperfractionated 1.4 Gy being given twice daily up to a total dose of 62.4–76.4 Gy. Radiotherapy was based on a planning CT scan. The gross tumor volume included the primary tumor and involved lymph nodes, and the planning target volume included the gross tumor volume with a margin of 1.0–1.5 cm. All patients were treated with 2 cycles of CCRT with a cisplatin-based regimen. The chemotherapy regimens used in this study were cisplatin/gemcitabine, cisplatin/docetaxel, cisplatin/vinorelbine, and cisplatin/pemetrexed as reported previously (9). These chemotherapy regimens were known to possess similar activity and effectiveness for treatment of NSCLC. After CCRT, patients without tumor progression underwent further chemotherapy with the same regimen, which was administered every 3 wk for a total of 2–4 cycles. Chemotherapy was changed to second-line regimens if progressive disease was present after 2 cycles of CCRT.
18F-FDG PET/CT Imaging
All patients fasted and rested for at least 6 h before undergoing PET/CT (Discovery LS PET/CT system; GE Healthcare). The uptake time was constant for the individual patient. At 45–60 min after intravenous injection of 370 MBq (10 mCi) of 18F-FDG, PET emission images were acquired from the level of the middle skull to the proximal thigh for 5 min per field of view, each covering 14.5 cm, at an axial sampling thickness of 4.25 mm per slice. CT data were collected in helical acquisition mode. PET images were reconstructed with CT-derived attenuation correction using the ordered-subset expectation maximization algorithm. The attenuation-corrected PET images, CT images, and fused PET/CT images displayed as coronal, sagittal, and transaxial slices were viewed on a Xeleris workstation (GE Healthcare). Pretreatment baseline PET/CT scans, as part of the initial staging, were done about 1–3 d before the start of CCRT (time point 1). The second whole-body PET/CT scan was recorded at the middle of CCRT (40 Gy of radiotherapy with 2 cycles of chemotherapy, time point 2) to exclude tumor progression locally or at distant sites. The mean time (±SD) from points 1 to 2 was 28 ± 3 d (Fig. 1). A commercial radiotherapy pallet was mounted on the PET/CT patient table to facilitate adequate positioning of the patient corresponding to the radiotherapy settings using standard immobilization devices.
Schematic of PET/CT and CT scans.
18F-FDG PET Image Analysis
The PET/CT images were analyzed by 2 experienced nuclear medicine physicians without knowledge of the patients’ history. Initially, 18F-FDG PET data were transferred into the workstation in DICOM format. Semiquantitative measurements of metabolic uptake in 18F-FDG–avid tumors after pretreatment and intratreatment scans were compared and evaluated for their potential to predict survival. The calculation methods for SUVmax, SUVmean, and MTV were described in detail in our previous study (9). The percentage decrease (Δ) in each of the parameters (P) between baseline (pre) and during treatment (intra) was calculated using the following formula: Eq. 1.A positive value indicated a reduction in that parameter after therapy, and a negative value indicated an increase. The thresholds for changes in SUVmax, SUVmean, and MTV defined by ROC curve analysis associated with short-term outcome were 37.2%, 41.7%, and 29.7%, respectively. For consistency with our previous study, these cutoffs were used in the analysis to separate the groups for OS.
Eq. 1.A positive value indicated a reduction in that parameter after therapy, and a negative value indicated an increase. The thresholds for changes in SUVmax, SUVmean, and MTV defined by ROC curve analysis associated with short-term outcome were 37.2%, 41.7%, and 29.7%, respectively. For consistency with our previous study, these cutoffs were used in the analysis to separate the groups for OS.
Endpoint and Statistical Analysis
After completion of treatment, all patients were followed up every 3 mo over the first 2 y and every 6 mo thereafter. The endpoint evaluated in this study was 2-y OS. OS was observed from the first day of treatment until death or last follow-up. The data were analyzed by SPSS version 17.0 (SPSS Inc.; IBM Co.). We present data as mean ± SD for central tendencies, as median followed by range in parentheses for skewed data, and as frequency and percentage for categoric variables. Cox regression analysis provided a univariate and multivariate analysis of the endpoint with selected prognostic factors including PET/CT parameters and other clinical variables. The level of significance of a series of prognostic factors was estimated using the log-rank test. Survival curves were generated using the Kaplan–Meier method. Differences were assumed to be significant when the P value was less than 0.05.
RESULTS
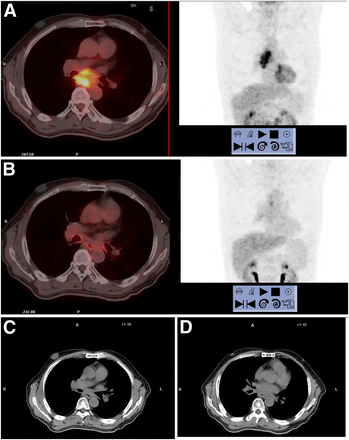
Figure 2 presents the 18F-FDG PET/CT and CT scans of a typical patient with responding tumor.
18F-FDG PET/CT and CT scans in typical patient with responding tumor: pretreatment 18F-FDG PET/CT (3 d before CCRT) (A); intratreatment 18F-FDG PET/CT (at 4 wk) (B); posttreatment CT (at 2 mo) (C); follow-up CT (at 2 y) (D).
Short-Term Outcome
Short-term outcome was assessed at 4 wk after treatment (62.4–76.4 Gy of radiotherapy and 4–6 cycles of chemotherapy) using the Response Evaluation Criteria in Solid Tumors without knowledge of the results of the PET studies (10). Of the 63 patients, 1 died from a heart attack and 1 died from tumor bleeding before response assessment by CT, and 8 patients did not finish the treatment because of economic or personal reasons. As a result, 5 patients with a complete response and 28 with a partial response were assessable for response, and the overall response rate was 62.3%. In contrast, 17 patients achieved stable disease and only 3 patients had progressive disease. Those 3 patients were excluded during the initial CCRT and were changed to second-line chemotherapy.
Survival Outcome
Minimum follow-up was 2 y. OS at 1 and 2 y was 83.0% (44/53) and 52.8% (28/53), respectively. Median survival was 24.0 mo (range, 9.0–47.0 mo): 26.0 mo (range, 24–47.0 mo) and 19.0 mo (range, 9.0–42 mo) for the surviving and deceased patients, respectively. During follow-up, 6 patients were alive without known recurrent disease or metastasis, and 12 patients had locoregional recurrence or metastasis but were still alive after salvage or palliative treatment. Seventeen of the deceased patients died of tumor recurrence; 11 died of the distant organ metastasis, including brain, liver, and lung; 6 died of both issues; and 1 died of radiation pneumonitis. Table 2 summarizes the outcomes for patients in this study.
Outcomes of 53 NSCLC Patients
PET Parameters and the Kaplan–Meier Curve
Table 3 shows changes in the parameters for pretreatment and intratreatment 18F-FDG PET/CT scans for all patients. The cutoffs of SUVmax, SUVmean, and MTV changes (37.2%, 41.7%, and 29.7%, respectively) determined by the previous study were used in the analysis to separate the groups. The Kaplan–Meier curves for OS at the cutoffs for the decrease in SUVmax, SUVmean, and MTV are shown in Figures 3⇓–5. For SUVmax, there was no statistical difference between the 2 groups using this cutoff to separate the 2 groups. However, survival curves for SUVmean and MTV were significantly different using the cutoffs to divide the dataset into 2 groups (both P < 0.001). Median OS was 37.5 mo and 2-y OS survival 75.0% (12/16) in patients with a decrease in SUVmean of more than 41.7%, compared with a median OS of 19.5 mo and a 2-y OS of 37.8% (14/37) in patients with a decrease in SUVmean of less than 41.7%.
Changes in Parameters of 18F-FDG PET/CT Scans
Kaplan–Meier curves showing OS depending on decrease in SUVmax. Data are median OS of patients with high (>37.2%, green) or low (<37.2%, blue) ΔSUVmax (27.0 mo vs. 22.0 mo, P = 0.765).
Kaplan–Meier curves showing OS depending on decrease in SUVmean. Data are median OS of patients with high (>41.7%, green) or low (<41.7%, blue) ΔSUVmean (37.5 mo vs. 19.5 mo, P < 0.001).
Kaplan–Meier curves showing OS depending on decrease in MTV. Data are median OS of patients with high (>29.7%, green) or low (<29.7%, blue) ΔMTV (36.5 mo vs. 18.0 mo, P < 0.001).
Cox Regression and Prognostic Factors
Cox regression analysis for OS was used to analyze the relationships between covariates of interest. The results of univariate analysis are summarized in Table 4. Among the 17 variables of univariate analysis, 6 variables were identified as having prognostic significance: decrease in SUVmax (P = 0.016), intratreatment SUVmean (P = 0.018), decrease in SUVmean (P < 0.001), intratreatment MTV (P < 0.003), decrease in MTV (P < 0.001), and short-term outcome (P < 0.001). Multivariate analysis included the 6 prognostic significance factors in univariate analysis. The results of multivariate analysis are shown in Table 5. Multivariate analysis by the Cox proportional hazards model showed that a decrease in MTV was the only independent prognostic factor for survival (P < 0.001; 95% confidence interval, 0.000–0.012).
Univariate Analysis with Cox Regression
Multivariate Analysis with Cox Regression
DISCUSSION
CCRT is a demanding strategy that has a high burden for the patient with NSCLC. Selection of patients up front who will benefit is difficult, and an early assessment of accurate prognosis could allow clinical decision making and treatment selection. PET/CT imaging has become an increasingly important component of staging, tumor localization for radiotherapy treatment planning, and assessment of treatment response in NSCLC (11,12). Our previous study investigated a group of NSCLC patients with repeated imaging after they had received approximately 40 Gy of CCRT, and short-term outcome was evaluated using the Response Evaluation Criteria in Solid Tumors at 4 wk after the end of treatment. Changes in SUVmax, SUVmean, or MTV allowed prediction of the short-term outcome of treatment. However, effect on OS was not presented at that time. As is well known, the ultimate objective of treatment is to improve the survival of cancer patients. The ability of 18F-FDG PET to separate patients with a good prognosis from those with a poor one in the early stage of treatment would be of help in deciding on the most appropriate treatment and particularly in stratifying patients for clinical trials. Therefore, we conducted a follow-up of these patients to determine whether 18F-FDG PET can also predict survival.
Various parameters are used in measuring changes in tumor glucose metabolic activity with 18F-FDG PET in response to cancer treatment. However, which of these provides the lowest variability among observers is still controversial. The semiquantitative parameter of 18F-FDG uptake that is currently most widely used for the assessment of therapeutic response in tumors is SUVmax (13,14). The most important concern with SUVmax is that a mildly active tumor may have a single hot pixel that may arise from random error rather than actual abnormal uptake in the body. To overcome random variations in SUVmax, SUVmean, which is calculated by averaging the SUVs generated from the entire tumor, can be used (15). More recently, MTV, which indicates the volume of metabolically active tumor, has been explored as a measure of metabolic tumor burden (16,17). For consistency with our previous study, we also used the SUVmax, SUVmean, and MTV of the primary tumor to predict survival in patients with locally advanced NSCLC treated with CCRT.
The prognostic value of 18F-FDG PET at diagnosis has been evaluated in several studies (18–21). These studies showed that pretreatment 18F-FDG PET not only improved patient staging but also provided prognostic information. Univariate analyses to determine a cutoff for SUVmax in the primary tumor to discriminate between a more or less favorable prognosis have ranged widely from 5 to 20 (4,22,23). Baseline whole-body MTV also has this kind of prognostic ability. Lee et al. performed the first study to show that baseline whole-body MTV measured semiautomatically was a statistically significant prognosticator in 19 patients with lung cancer and was better than SUVmax and SUVmean (5). In a larger study by the same author including 328 surgical and nonsurgical patients, baseline whole-body MTV was a prognostic marker independent of stage, treatment intent, patient age, sex, and tumor histology (24). However, baseline SUVmax (P = 0.612), SUVmean (P = 0.999), and MTV (P = 0.105) had no prognostic value in our population or in other studies (25,26). The cause of this heterogeneity may be differences in treatment methods, disease stage, enrolled populations, acquisition protocols, or institutional-based technical factors. In contrast, our study had a homogeneous patient cohort in which all patients had the same tumor stage (III) and were treated by the same therapeutic protocol (CCRT) to objectively assess the prognostic value provided by 18F-FDG parameters.
The prognostic value of 18F-FDG PET has not been studied as thoroughly after induction treatment as at diagnosis. There is increasing interest in determining the prognostic value of intratreatment 18F-FDG PET, especially early in the course of first-line therapy. A study investigated the possibility of early response assessment based on 18F-FDG uptake during radiotherapy with respect to OS in patients with NSCLC. 18F-FDG PET was performed before radiotherapy and was repeated in the second week of radiotherapy for 34 consecutive lung cancer patients. The results showed that a decrease in metabolic activity of the primary tumor by as early as the second week of treatment was predictive of survival (25). Our previous study proved that 18F-FDG PET/CT can differentiate responders from nonresponders early in the course of CCRT. The thresholds for changes in SUVmax, SUVmean, and MTV determined by ROC curve analysis in the study were 37.2%, 41.7%, and 29.7%, respectively. For consistency with that work, we still use those thresholds as cutoffs to analyze survival in patients with long-term follow-up. When a cutoff of a 37.2% reduction in initial 18F-FDG uptake is applied as a criterion for metabolic response, SUVmax cannot differentiate survival between the 2 groups, but both SUVmean and MTV can separate the 2 groups successfully. Although a decrease in SUVmean can separate the 2 groups with the Kaplan–Meier curve, multivariate Cox regression analysis showed that the only significant parameter for OS was a decrease in MTV. Recently, however, Vera et al. reported that the SUVmax at PET at the middle of radiotherapy (mean dose, 43 Gy of radiotherapy) was the only variable predictive of death or tumor progression at 1 y in NSCLC patients who underwent radiotherapy with or without concomitant chemotherapy (26). This finding diverges from our conclusion but may have several explanations: first, 77% of the patients in the study of Vera et al. received induction chemotherapy and 27% of those did not receive CCRT, but all our patients underwent CCRT without induction chemotherapy. Second, the stages of their patients varied from IA to IIIB whereas only patients with stage III disease were enrolled in our study.
MTVs generated from the region-growing algorithm are 3-dimensional measures and incorporate both tumor volume and metabolic activity. Therefore, MTV reflects change throughout the entire tumor mass and may be a more sensitive method of detecting change than a single-pixel value. This characteristic may explain why MTV predicted survival more accurately than SUVmax and suggests that although the maximally active portion of the tumor may represent the more aggressive part, the maximally active portion has less impact on outcome, possibly because it shows a greater response to treatment. Furthermore, by using MTV as our metric of volumetric tumor burden, we can take advantage of the high signal-to-background ratio of 18F-FDG PET, making possible rapid semiautomatic computer-based methods that limit interobserver variability. Although the prognostic value of SUVmax may be inferior to MTV, we believe that SUVmax should still be considered in monitoring disease response. This is one of the first studies showing that repeated 18F-FDG PET early during CCRT has added value by being a prognostic factor for survival of NSCLC patients (27,28). A decrease in the MTV of the primary tumor correlates with higher long-term OS.
CONCLUSION
Our data suggest that the MTV of the primary tumor has the potential to become a valuable prognostic biomarker for survival outcome in NSCLC patients. A decrease in MTV according to 18F-FDG uptake by the primary tumor correlates with higher long-term OS. On the basis of early treatment response assessment using changes in MTV, it might be possible to improve management in NSCLC patients by avoiding ineffective CCRT and to select patients who require more aggressive treatment to improve their outcome.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by grant BS2013YY057 from the Research Award Fund for Outstanding Young Scientists funded by the Department of Science and Technology of Shandong Province, China. No other potential conflict of interest relevant to this article was reported.
Footnotes
↵* Contributed equally to this work.
Published online Sep. 11, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication May 9, 2014.
- Accepted for publication August 4, 2014.