Abstract
Hypoxia, a condition of insufficient O2 to support metabolism, occurs when the vascular supply is interrupted, as in stroke or myocardial infarction, or when a tumor outgrows its vascular supply. When otherwise healthy tissues lose their O2 supply acutely, the cells usually die, whereas when cells gradually become hypoxic, they adapt by up-regulating the production of numerous proteins that promote their survival. These proteins slow the rate of growth, switch the mitochondria to glycolysis, stimulate growth of new vasculature, inhibit apoptosis, and promote metastatic spread. The consequence of these changes is that patients with hypoxic tumors invariably experience poor outcome to treatment. This has led the molecular imaging community to develop assays for hypoxia in patients, including regional measurements from O2 electrodes placed under CT guidance, several nuclear medicine approaches with imaging agents that accumulate with an inverse relationship to O2, MRI methods that measure either oxygenation directly or lactate production as a consequence of hypoxia, and optical methods with NIR and bioluminescence. The advantages and disadvantages of these approaches are reviewed, along with the individual strategies for validating different imaging methods. Ultimately the proof of value is in the clinical performance to predict outcome, select an appropriate cohort of patients to benefit from a hypoxia-directed treatment, or plan radiation fields that result in better local control. Hypoxia imaging in support of molecular medicine has become an important success story over the last decade and provides a model and some important lessons for development of new molecular imaging probes or techniques.
Oxygen is an essential nutrient for mammalian cells because of its role as the terminal electron acceptor in oxidative phosphorylation. Supplied through breathing, O2 dissolves in plasma and tissues and is carried throughout the body as oxyhemoglobin, O2Hb, which releases O2 when it is needed for mitochondrial respiration. It is so essential to the well-being of cells that intricate control mechanisms have evolved to maintain a sufficient supply of O2 for the mitochondria. This control system can adjust blood flow, blood vessel dilation, or oxygen extraction fraction to maintain a life-sustaining level of O2.
In the brain, oxygen physiology changes rapidly in response to stimulation by simple tasks such as visual or auditory input or language function, and this rapid response is monitored in functional mapping of the brain using PET (1) or fMRI (1,2). These processes also change in response to stroke and have been studied with a protocol using 15O-O2 plus its metabolite, 15O-H2O, and the measurement of vascular volume using inhaled 15O or 11C-labeled CO, which binds to red cells as COHb (3,4). A qualitative version of this test has been used to select appropriate subjects for the carotid occlusion surgical study (5,6).
When otherwise healthy cells are suddenly deprived of sufficient O2 to maintain oxidative phosphorylation, the cells die either by necrosis or programmed cell death, apoptosis. This phenomenon is called hypoxia and it happens, for example, in stroke, myocardial infarction, or as a consequence of poor perfusion in diabetic limbs or arthritic joints. Cancer cells often respond to hypoxia in a different way, as will be discussed in the next section.
Hypoxia is therefore a phenomenologic concept. There is no specific value of O2Hb concentration, % Hb saturation by oximetry, or tissue partial pressure, PO2 measured with electrodes, that results in a transition from normoxia to hypoxia. Of course, PO2 gradients also exist from the point of supply (vasculature) to consumption (mitochondria). The biologic consequences of hypoxia depend on duration and the needs of individual cells; speed is critical in reestablishing perfusion after a stroke or infarction.
Clearly, the ability to identify hypoxia has implications in a wide range of medical settings. How can this best be accomplished? The goal is to develop a positive image of the absence of something, namely O2, because the critical effect happens when O2 levels are too low to satisfy the metabolic demand. Oxygen-sensitive electrodes, when properly calibrated, can directly measure PO2 in units of mm Hg (7–10), but the signal is very small in the range of cell-threatening hypoxia. This technique has the practical disadvantages of requiring CT or ultrasound-guided placement, and accessible tissue beds limit sampling, and it invades and damages tissues. There are fluorescent needle probes where the optical signal is quenched by O2 so that the signal increases as O2 values fall (11–13). This resolves one limitation of electrodes, but it still requires accessible sites. Fluorescent techniques also have much lower oxygen consumption than do electrodes, which may be particularly crucial at low PO2.
MRI provides a useful way to measure hypoxia. Absolute PO2 can be measured on the basis of fluorocarbon reporter molecules. These may be introduced by direct intratumoral injection and they provide measurements consistent with electrodes (presumably interstitial PO2). A major advantage over electrodes is that maps of regional PO2 may be measured at 50–150 individual locations simultaneously. Moreover, once the reporter molecule has been introduced, sequential PO2 maps may be generated to reveal changes in oxygenation with respect to interventions, such as hyperoxic gas breathing or vascular targeting agents (14,15). Because of a lack of human MRI systems with a 19F MRI capability, 19F oximetry measurements have not yet been attempted in patients.
Blood Oxygen Level Dependant (BOLD) MRI is an imaging technique that distinguishes paramagnetic deoxy-Hb from O2Hb. Appropriate T2*-weighted imaging reveals changes in vascular oxygenation. A limitation is that it is also sensitive to changes in Hb concentration, which may result from alterations in vascular volume and flow as well as interconversion of oxy- and deoxy-hemoglobin. Therefore, this technique provides qualitative assessment of changes in oxygenation rather than quantitative measurements. This technique is widely used for functional brain mapping (1,16,17), where it is thought to primarily reflect changes in flow. It is starting to be applied to tumor studies. BOLD is particularly responsive to oxygen manipulation accompanying hyperoxic gas breathing as a simple way to ameliorate hypoxia (18,19).
Dissolved molecular oxygen is itself paramagnetic and shortens NMR spin lattice relaxation time. This has been exploited to explore changes in tissue oxygenation accompanying hyperoxic gas breathing, where T1-weighted signals increase with improved oxygenation (20), but since many factors influence T1 it is unlikely to provide a quantitative measure of PO2.
There are biologic sequelae of hypoxia that are amenable to imaging. For example, prolonged hypoxia can lead to increased lactate in tissues and 1H MRI can be used to image lactate. The redox state of nonprotein thiols, such as glutathione, is altered in hypoxic cells, and this can cause some radiopharmaceuticals to accumulate in hypoxia. A similar phenomenon can be measured when hypoxia results in altered adenine nucleotide redox state, NADH or NADPH. All of these tests are measuring a downstream consequence of hypoxia, and they often do not instantly return to normal after an adequate O2 supply has been established.
HYPOXIA IN TUMORS
When otherwise healthy cells are acutely deprived of O2, they invariably die. Tumor cells are gradually exposed to chronic hypoxia as they outgrow their vascular supply, and this can lead to an adaptation that has a negative effect on their response to treatment. If the tumor is not well perfused, chemotherapeutic drugs do not have good access to each tumor cell. Furthermore, tumor cells rapidly adapt to hypoxia by slowing their growth rate, and conventional chemotherapy generally is toxic to cells at a level that is proportional to proliferation. Most chemotherapeutic drugs are essentially antiproliferation agents rather than specific anticancer agents so that when cells enter a resting phase in their cycle, they are not sensitive to these cytotoxic agents.
Ionizing radiation is a strategy for killing proliferating cells that does not rely on vascular delivery because the radiation field is homogeneous. But the cytotoxicity of ionizing radiation depends on the level of intracellular O2. Gray observed over 50 y ago that about a 3 times higher dose of photon irradiation is required to kill O2-deprived cells than for well-oxygenated cells (21). Ionizing radiation damages DNA in several ways, most commonly by double-strand breaks from secondary radical products in the vicinity of the DNA. When the primary radical reacts with O2, the damage is rendered permanent, whereas reaction with small thiols can repair the radical damage. This oxygen enhancement ratio (OER) transitions from 1 to 3 in the range of 2,000–5,000 ppm of O2, below 5 mm Hg PO2. Clinical trials of oxygen-mimetic radiosensitizers plus radiotherapy did not result in a significant improvement in outcome, perhaps because systemic drug toxicity limited the dose (22), although a recent meta-analysis of 50 trials involving over 7,000 patients showed a significant, albeit small, improvement in local control for head and neck cancer (23). A follow-up meta analysis of over 10,000 patients in 83 randomized trials based on nitroimidazoles or other oxygen-modifying procedures such as breathing hyperoxic gas showed only minor benefit and may be attributed to the inability to stratify patients based on hypoxia and select patients who might benefit from adjuvant interventions (24).
Radiation oncologists have devised numerous strategies to overcome the cure-limiting consequences of hypoxia, including hyperbaric oxygen, transfusion to increase Hb levels, blood substitutes that are better carriers of O2, and others, but with little success (23,24). In fact the oxygen mimetics described in the next section as the basis for radiopharmaceutical imaging were one of the more effective strategies for combating tumor hypoxia (25). During the 1970s, the biochemistry of nitroimidazoles, small molecules with the potential to overcome the assumed mechanism of radiation resistance, was studied. When chemists started developing hypoxia imaging strategies, it was with the goal of identifying 3 factors in cancer biology that were thought to limit response to treatment: decreased blood flow and hence delivery of drugs, decreased proliferation and hence fewer cycling cells, and absence of the OER in these tumors. One of the first clinically significant contributions to radiation therapy from hypoxia imaging was the report of Rasey and Koh that oxygenation in some but not all lung tumors was returned to normal after the first few radiation treatments (26).
At the same time that these imaging studies were starting to be used in clinical research, several investigators were using O2 electrodes to measure PO2 in accessible tumors, most commonly cervix (27), head and neck (28), and breast (29). These electrode studies became more convenient with FDA approval of the Eppendorf PO2 Histograph (7,30), which could be used under CT guidance to collect a histogram of 50–80 PO2 measurements along 5 or 6 tracks to obtain an overall impression of the oxygen status of a tumor. In all of the tumor types that were studied, these results showed poorer response when an appreciable fraction of the PO2 readings were below 7–10 mm Hg. These patients had poorer local control and more metastases. By promoting metastatic spread, hypoxia can even limit cure by surgery in uterine cervical carcinoma (27,31,32) and soft-tissue sarcomas (33).
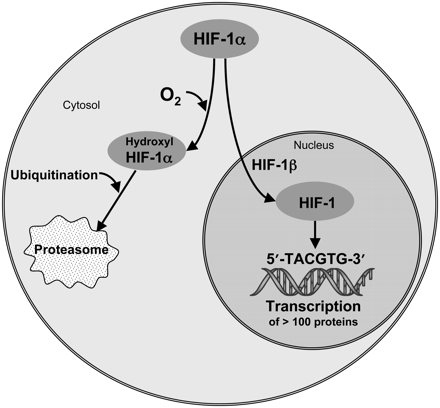
During the 1990s, the working model of the biologic significance of hypoxia in tumors became a lot more complicated. Semenza (34,35) worked out the detailed biochemistry involved in the molecular sensor of oxygen deprivation. His laboratory implicated hypoxia inducible factor 1 (HIF-1), as the transcription factor that leads hypoxic cells to up-regulate more than 100 proteins that promote survival and increased aggressiveness of hypoxic tumor cells. The mechanism is elegant in its simplicity. Briefly, mammalian cells along the whole evolutionary ladder constitutively express a protein that, when adequate O2 is present, becomes hydroxylated at several proline residues and this leads to ubiquitination and proteasomal degradation (Fig. 1). However, when O2 is absent this molecule survives as HIF-1α and translocates to the nucleus, where it forms a heterodimer with HIF-1β. The heterodimer, HIF-1, is the transcription factor that is recognized by at least 100 hypoxia response element sites on chromosomes. These sites are all characterized by the minimal motif of 5′-TACGTG-3′ of target genes, and they activate transcription of at least 100 proteins to support survival of the cancer cell (36,37). HIF-1 binding can also be mimicked by several oncogenic signaling pathways that bind to the same sequence, such as myc, even under normoxic conditions (36,37). Both insulin and insulinlike growth factor 1 induce HIF-1 activity, independent of hypoxia (38).
Schematic of HIF-1α pathways. HIF-1α either senses O2 and is degraded (left pathway) or survives in absence of O2, heterodimerizes, and binds to hypoxia response element domains (right pathway) to transcribe numerous proteins.
Table 1 is an abbreviated list of the HIF-1 transcription products adapted from Semenza (35). It includes proteins that are essential for glycolysis, a source of energy that does not require O2 and can take place under aerobic or anaerobic conditions. Other proteins promote growth and neovascularization or induce mutations in p53 that thwart apoptosis. Still others promote metastatic spread by up-regulating proteolytic enzymes that degrade extracellular matrix (37) and permit cells to break away from the primary tumor as well as molecules that down-regulate the immune surveillance system (39). In summary, the consequences of HIF-1 are uniformly directed toward survival of the tumor and lead to a poor response to cancer treatment for reasons that go far beyond the simplistic model of hypoxia being associated with removing cells from the most sensitive part of the cell cycle, compromising access of drugs to the cancer cells, or minimizing the OER. The importance of imaging hypoxia as a resistance factor leading to poor treatment outcome has much broader implications than were appreciated when hypoxia imaging was first developed in the 1980s (40–46).
Genes That Are Transcriptionally Activated by HIF-1
RADIOPHARMACEUTICAL APPROACH TO MEASURING HYPOXIA
The best way to measure hypoxia would be with a probe that competes directly with intracellular O2, one in which the indicator was not trapped when O2 supply was keeping up with demand but was retained when O2 supply was inadequate to accommodate all of the electrons being produced in the electron-transport chain (Fig. 2A). Furthermore, because of the coupling between blood flow and hypoxia, the probe should be delivered to all cells equally and independent of vascular flow. There is a well-studied class of molecules that meet these criteria, the oxygen mimetics that were once evaluated as an adjunct to increase the cytotoxicity of radiation therapy. Chapman suggested the potential of imaging using radiolabeled nitroimidazoles (40) and he and others (42,47–49) showed with microautoradiographs that 14C-labeled derivatives of N-alkyl-2-nitroimidazoles were trapped in cells that were hypoxic but still had electron-transport activity; that is, they were still alive. The necrotic center of tumors did not accumulate the 14C, and regions of tumor that were within 100–200 μm of a vascular supply and were therefore well oxygenated also failed to accumulate tracer.
(A) Molecular oxygen is terminal electron acceptor in mitochondrial respiratory chain. Electrons mostly react with O2 to form H2O in a process catalyzed by cytochrome c oxidase, complex IV. Some e− escape and combine with O2 to form superoxide radical anion, which reacts with superoxide dismutase (SOD), which is abundant in anaerobic organisms; alternatively, reactive oxygen species (ROS) oxidize critical biomolecules, leading to cellular dysfunction and death. H2O2 is substrate for catalase and peroxidase enzymes and eventually produces more H2O. (B) Mechanism of reduction and intracellular retention of nitroimidazoles involves buildup of steady-state level of 1 e− radical anion, which reacts preferentially with O2 if it is present to return tracer to its original form. If O2 is not present, it accepts another e− to produce intermediate that is sequentially reduced to highly efficient alkylating agent, RNH2, resulting in cellular retention of labeled tracer.
The mechanism by which 2-nitroimidazoles are reduced and retained in hypoxic tissues is now reasonably well understood (Fig. 2B). Several different derivatives of this structure have octanol/water partition coefficients that are close enough to 1 so that they are freely diffusible and homogeneously distributed in the body within an hour after injection at levels that reflect the tissue partition coefficient and maintain a volume of distribution essentially equal to that of total body water (50). This characteristic can be visually appreciated by viewing Figure 3 in the article by Lee and Scott (46). They are less than 5% protein bound, allowing efficient transport from blood into tissues. Therefore, their biodistribution is not complicated by reduced blood flow (42,51,52). The nitro functionality has an affinity for electrons, although less so than O2. This means that a terminal e− from the respiratory cycle can bind to the −NO2 group to form the radical anion, −NO2−. A small but steady-state level of these radical ions exists wherever carbon substrates, for example, glucose or fatty acids, are being metabolized by oxidative phosphorylation to produce electrons. If O2 is present in the cell in a concentration sufficient to compete for these extra electrons, the O2 can accept the electron from the nitro radical, forming conventional terminal electron products and returning the nitroimidazole radiopharmaceutical to its parent state. If, on the other hand, another electron reacts with the nitro radical anion first, making the 2-electron reduction product, O2 can no longer reverse the process. Then the labeled nitroimidazole goes on to form an alkylating agent that reacts indiscriminately with intracellular macromolecules; it is irreversibly trapped (53). The reduction of the nitro group on the imidazole ring is accomplished by tissue nitroreductases that are plentiful and do not represent a rate-limiting factor (54). Furthermore, the PO2 where nitroimidazoles are retained in cultured cells is in the same range as that where the OER is observed (Fig. 3).
Uptake (arbitrary units) of 3H-FMISO increases by greater than 20-fold at low PO2 (left axis and solid line). This process occurs at approximately same O2 pressure at which oxygen enhancement effect (right axis and dashed line) decreases from its normoxic value of about 3 to its hypoxic value of about 1. OER is ratio and thus has no units. These experiments involved cells in cultures.
This is the molecular basis for an imaging agent that is trapped at a level proportional to the intracellular demand for O2 and is not limited by blood flow, meeting the design requirements for a hypoxia imaging agent. 2-Nitroimidazoles are most commonly labeled with PET radionuclides such as 18F or 124I (42,55) but can also be labeled with single-photon emitters, such as 123I (45,56) or even 99mTc (45,57,58), or with multiple 19F atoms for detection by MRI (59,60). Because of their long history, this review provides substantial detail on labeled nitroimidazoles for PET, especially fluoromisonidazole, the most widely studied hypoxia imaging agent (46).
PHARMACOLOGY AND BIOCHEMISTRY OF NITROIMIDAZOLES
Nitroimidazoles have been studied extensively as oxygen mimetics designed to increase the cytotoxicity of ionizing radiation in tissues with low O2 concentration. Much of this research took place in the 1970s and 1980s and has been critically reviewed by several authors (61,62). Metabolism of misonidazole (MISO) is similar to that of fluoromisonidazole (FMISO). Several intracellular nitroreductase enzymes (including xanthine oxidase, lipoxygenases and NADPH oxidases) lead to the single-electron product that is the basis for the imaging strategy. There are other metabolic reactions that occur in vivo and lead to products that contribute to background but do not indicate hypoxia signal. In vivo under normal oxygen tension, MISO is metabolized primarily in the liver to its desmethylated derivative, but this reaction cannot occur with FMISO because it lacks the methoxy substituent. From 7% (humans) to 14% (mice) is conjugated to the glucuronide and less than 5% is reduced to aminoimidazole. Substantial amounts of MISO are recoverable in feces, and fecal anaerobic bacteria can reduce misonidazole in the absence of oxygen (42). Both FMISO and MISO have similar body clearance, and their metabolites are primarily excreted in the urine (42,63,64). No measurable amounts of defluorinated metabolites from FMISO were found in plasma or urine at 4 h after injection (51).
Our knowledge of the toxic effects of 2-nitroimidazoles in humans is based on misonidazole, a close analog of FMISO. The plasma half-life of FMISO in humans is similar to MISO as they have similar sizes and partition coefficients and both are cleared primarily through the kidneys. The therapeutic potential of nitroimidazole radiosensitizers has been studied in over 7,000 patients in 50 randomized trials as reviewed by Overgaard (23). Oral MISO was the agent in 40 trials involving 5,377 patients. The maximum doses used were 4 g/m2 in a single dose and 12 g/m2 as a total dose. Clinical studies using multiple dosing of MISO have reported that peripheral neuropathy with a latency period of several weeks was the manifestation of toxicity that became dose-limiting with daily oral doses of over 3 g/m2 (65). Limiting the total dose and giving no more than 2 doses in 1 wk minimized neuropathy. Nausea, vomiting, skin rashes, ototoxicity, flushing, and malaise have also been reported (66). Patients receiving up to 140 mg/kg tolerated the drug well. Neuropathies were generally, but not always, reversible when the drug was discontinued. There have been 2 fatalities attributed to misonidazole (62). Both patients had advanced malignant disease and died in convulsions; one patient received 51 g orally in 17 d, 6 fractions, and the other had 16 g in 3 d, 2 fractions.
Projections based on the interspecies pharmacokinetic models of Paget (67) rely on similarities among related chemical entities. The octanol/water partition coefficients for MISO and FMISO are both 0.4; the LD50s in adult male BALB/C mice for MISO and FMISO are 1.8 mg/g and 0.9 mg/g, respectively (50). Using the relative toxicity factors from Paget of 1.0 for mice and 9.8 for humans, the projected LD50 values for a 70-kg person are about 12.5 g for MISO and 6.5 g for FMISO. This MISO value is consistent with the findings from the early clinical trials.
The dose to humans in initial FMISO nuclear imaging protocols was as high as 1 mg/kg, approximately 0.1% of the calculated LD50. Total patient imaging dose for the current radiopharmaceutical formulation is less than 15 μg. That the drug has no adverse effects at these doses can be confidently stated as a result of the 5,377 patients treated with MISO (23) and the growing literature reports of imaging studies with several nitroimidazole derivatives for nuclear, magnetic resonance and immunohistochemical detection.
Fluoromisonidazole: Preclinical Studies
Uptake of FMISO by multicellular spheroids, aggregates of cells that grow in culture and mimic small tumors, provided a visual and a quantitative measure of hypoxia. Autoradiographs of 0.8-mm V79 spheroids after 4 h of incubation with 3H-FMISO revealed heavily labeled cells in an intermediate zone between the well-oxygenated periphery and the necrotic center (Fig. 4) (68). The relationship between O2 concentration and 3H-FMISO binding was also studied in monolayer preparations of isolated adult rat myocytes (69). Under anoxic conditions, 3H-FMISO binding after 3 h was approximately 25-fold greater than normoxic controls. This level of binding was reduced to 40% at a PO2 of ∼4 mm Hg. 3H-FMISO uptake was not influenced by glucose or thiol concentrations or by cellular pH, potential confounding variables in the tumor microenvironment (42,54).
Microautoradiograph of EMT6 spheroid exposed to 3H-FMISO in normoxic cell culture medium. Tritium appears as white speckles. There is no radioactivity in necrotic center, where cells have died, and there is no electron transport. Also, there is no more activity in peripheral ∼150-μm layer than there is in medium. Activity is uniformly intense in hypoxic donut (bisected by white line), where cell distance from oxygenated medium exceeds O2 diffusion limit.
Regional variation in hypoxia within a single tumor has been observed and might result from differences in delivery of the radiopharmaceutical as well as PO2. To investigate the ability to separate these 2 effects, relative blood flow was measured in tumors and in several normal tissues of mice by sacrificing the mice a few seconds after injection of the freely diffusible blood flow tracer, 14C-iodoantipyrine, that has the same partition coefficient as FMISO. Relative flow was compared with distribution of FMISO injected 4 h earlier. Each tumor was dissected into several pieces representing central and peripheral regions, and multiple samples were taken from several normal organs (42). The relative blood flow measurements confirmed that the tumors had generally low flow and the highest flows were in lung and kidney. In individual tumors there was no correlation between regional flow and regional FMISO retention. The same lack of correlation was also found in normal tissues. The conclusion that FMISO uptake is independent of blood flow, even at very low flow, has been confirmed in larger animals (52,70).
Fluoromisonidazole and Related Nitroimidazoles: Clinical Studies
18F-fluoromisonidazole [1-(2-nitroimidazolyl)-2-hydroxy-3-fluoropropane; 18F-FMISO], is the most widely used PET agent for mapping regional hypoxia. It was originally prepared by reacting 2-nitroimidazole with the volatile precursor, 18F-fluoroepihydrin (71,72), but this procedure was soon replaced by a more convenient nucleophilic displacement method (Fig. 5) (73) that is procedurally similar to preparing 18F-FDG. This method is now the basis for an IND sponsored by NCI-CIP for clinical trials using 18F-FMISO (74). Typical radiosynthesis yields are 3 GBq starting from 11.1 GBq of 18F-fluoride in target water. The synthesis, including semipreparative high-performance liquid chromatography (10 μm of C18 eluted with 5% ethanol in water), takes about 35 min, and the product is collected in 5% ethanol (US Pharmacopeia) in sterile water for injection (US Pharmacopeia). In our hands, the specific activity is typically 250 GBq/μmol, and a patient injection of 260 MBq always involves the administration of less than 15 μg of 18F-FMISO, typically 0.2 μg. Although the initial synthesis provided an optically active diastereomer (72), the product from the racemic precursor (75) has the same biodistribution characteristics as the pure enantiomer (76).
Schematic for radiochemical synthesis of 18F-FMISO. EtOH = ethanol; HPLC = high-performance liquid chromatography; MeCN = acetonitrile; OTHP = O-tetrahydropyranyl; OTs = O-toluene sulfonyl.
The 18F-FMISO imaging protocol is similar to a bone scan protocol, with which cancer patients are generally familiar. Patients do not need to fast before the study. The desired dose of 18F-FMISO is injected, and the patient is asked to return to the nuclear medicine clinic in about 1.5 h An attenuation-corrected image is collected beginning about 90–120 min after the radiopharmaceutical was injected and for sufficient time to collect enough emission events to provide a good-quality image, typically 20 min. This timing is selected to ensure that the tracer has equilibrated proportionally to the partition coefficient in blood and tissue. A venous blood sample is drawn during the imaging session, and the static image data in units of Bq/cm3 are normalized to the 18F concentration in the blood, Bq/mL, to provide a normalized map of tissue-to-blood ratio, T/B, for each pixel in the image. Because 18F-FMISO partitions nearly equally between octanol and water, normoxic tissues have T/B pixel values of almost 1.0. When the O2 supply is adequate, most tissues have the same level of 18F as is in the blood. Because of the partitioning mechanism, whole-body clearance of 18F-FMISO is slow. Thus, the contrast is not high in raw 18F-FMISO images, and 18F-FMISO PET has been criticized for this reason. However, the fact that pixels with a T/B of less than 1 are reliably normoxic allows for unambiguous visualization of regions that are truly hypoxic.
The hypoxic part of a tumor can be characterized by the maximum T/B value or by the total number of pixels with T/B greater than some threshold. These pixels can be summed and multiplied by the known mL/pixel to calculate a hypoxic volume, HV. Although these 2 parameters should be expected to covary, the T/Bmax indicates the greatest level of hypoxia in the tumor whereas the HV indicates the total volume of tumor that is hypoxic. The relative predictive value of these 2 parameters is under investigation.
The uptake of 18F-FMISO in normal human tissues has been measured and used to estimate the radiation-absorbed dose (77). All tissues demonstrated a rapid uptake phase followed by exponential clearance, and all tissues received a similar radiation dose, reflecting the similarity of biodistribution to that of water (50). Estimated total-body absorbed dose for a 70-kg man injected with 3.7 MBq/kg is 0.013 mSv/MBq; for a woman it is 0.014 mSv/MBq. The radiation exposure from 18F-FMISO is equal to or lower than those from other widely used radiopharmaceuticals.
Positron emission scanning with 18F-FMISO has been studied widely over the past 15 y. The general conclusion from these studies is that 18F-FMISO PET identifies hypoxic tissue that is heterogeneously distributed within human tumors (42,78) and promises to help facilitate image-directed radiotherapy (79,80) and clinical trials of new hypoxia-selective cytotoxins (81) as ways to circumvent the cure-limiting effects of tumor hypoxia. In addition, 18F-FMISO has identified a discrepancy between perfusion, blood–brain barrier disruption, and hypoxia in brain tumors (82) and a lack of correlation between 18F-FDG metabolism and hypoxia in several types of malignancies (83–87). Hypoxic tissue does not correlate either with tumor volume or vascular endothelial growth factor (VEGF) expression (83,88). 18F-FMISO imaging was used to identify postradiotherapy tumor recurrence by differential uptake of tracer. The standardized uptake value (SUV) ratio between recurrent tumor and muscle was greater than 1.6 and between tumor and normal mediastinum was greater than 2.0 (89). Only one study has concluded that 18F-FMISO was not “feasible for the detection of tumor hypoxia in human soft-tissue tumors” (90), although hypoxia imaging was developed to characterize tumors, not for detection.
Nuclear medicine methods for imaging hypoxia in patients with cancer have been reviewed in several publications (42–46,78). Although its clinical role is still being assessed, 18F-FMISO has been established as a robust radiopharmaceutical for obtaining images to quantify hypoxia using PET, and it remains the single most commonly used agent for imaging hypoxia. Although its biodistribution properties do not result in high-contrast images, they result in an image at about 2 h after injection that unambiguously reflects regional PO2 in the range where it is clinically significant. In human metastatic neck lymph nodes, comparison of the 18F-FMISO tumor-to-muscle uptake ratio at 2 h and Eppendorf PO2 histography found an average to high correlation; not surprisingly, no correlation was found with 18F-FDG (85). Studies have shown that a level of hypoxia higher than the median value is predictive of poor locoregional control and a higher rate of metastatic spread. In head-to-head comparisons of 18F-FDG and 18F-FMISO imaging, 18F-FMISO was a stronger predictor of outcome than 18F-FDG and it had independent value (91). A significant correlation was found between hypoxic tissue identified by 18F-FMISO and both pimonidazole and carbonic anhydrase IX, CA9, detected by immunohistochemical staining techniques (92), although other investigations have questioned the validity of CA9 as a reference standard for hypoxia (93). A recent critical review of 18F-FMISO PET provides a useful summary of results from several clinical studies involving over 300 patients (46). It concluded that 18F-FMISO has been validated in multiple studies and is “gaining increasing importance for its potential to predict response to treatment and provide prognosis in a wide range of pathologic processes.”
Several alternative nitroimidazole derivatives have been described with the goal of a possibly more optimal partition coefficient and therefore faster clearance properties, less nonspecific retention, or fewer metabolites. Motivation for developing new nitroimidazole radiopharmaceuticals came from a report of metabolites in the urine and plasma of mice injected with 18F-FMISO (94) and a desire to accelerate whole-body clearance of the radiopharmaceutical. Although the mouse study showed 5% metabolites for 18F-FMISO after 2 h, no time course for metabolism has been reported and the total amount in plasma at 2 h, inferred from the blood clearance data in the same paper, suggests that under the worst case less than 5% of the total injected dose was plasma metabolite. Metabolite levels have not, to the best of our knowledge, been reported in human studies sufficient to require a correction of the input function for metabolism.
Several alternative reagents are summarized in Figure 6 and Table 2 and have been evaluated as imaging agents in single-center pilot studies (45). A study from Finland reported using 18F-fluoroerythronitroimidazole (18F-FETNIM) to evaluate 10 patients with head and neck squamous cell cancer at doses of about 370 MBq without adverse effects (95). FETNIM was found to be equivalent to 18F-FMISO both in correlation with oxygenation status and in tumor-to-muscle uptake ratios in mice with implanted C3H mammary carcinomas (96). Other agents, fluoropropylnitroimidazole and fluorooctylnitroimidazole, have not proved as useful in visualizing hypoxic tissue (97), probably because of their greater lipophilicity than 18F-FMISO.
Structures of 2-nitroimidazole (2NIM), which are either secondary alcohols or amides. See Table 2 for more information.
Alkyl Substituents Affecting Partition Coefficients of 2-Nitroimidazole Imaging Agents
A derivative that is more hydrophilic than 18F-FMISO, 18F-fluoroazomycin-arabinofuranoside (18F-FAZA), had been recommended for further study (98,99) and shows considerable clinical promise. 18F-FAZA and 18F-FMISO were compared in 2 murine tumor models, and the tumor-to-muscle and tumor-to-blood ratios were higher for 18F-FAZA at all times, reflecting its somewhat faster clearance (100). A related derivative has been proposed, 124I-iodoazomycin galactoside, and was an effective imaging agent in some animal studies (101). The long nuclear half-life of 124I allows imaging at 24–48 h after administration, a time when much of the body background has cleared. Although this might be a way to minimize the effect of blood flow on hypoxia images, the requirement that patients return a day later would be a practical deterrent.
18F-fluoroetanidazole (18F-FETA) is yet another 2-nitroimidazole that shows promise as a hypoxia imaging agent. This molecule was first developed to circumvent some of the metabolic side reactions of 18F-FMISO in vivo where most of the 18F in the urine was not 18F-FMISO (94). A careful biologic evaluation of 18F-FETA found that this second-generation agent had potential advantageous physicochemical properties. Most notably it was stable to nonhypoxic degradation in vivo (102). Tumor retention of 18F-FETA reflected tissue PO2 in the same range as with18F-FMISO, 1–10 mm Hg. Its overall clinical value remains undefined at this time.
The more highly fluorinated 2-nitroimidazoles that were first developed for 19F NMR studies, described later, have been radiolabeled with 18F and evaluated as PET agents. These molecules are fluoroalkyl acetamide derivatives and have been abbreviated EF1 (103), EF3 (104), and EF5 (105). Within this family, EF5 has been thoroughly validated using drug adducts that can be detected by immunohistochemistry. A multicenter clinical trial to test EF5 as a hypoxia imaging agent is about to begin. Although there have not been any direct comparisons with 18F-FMISO PET, comparisons with pimonidazole and pharmacokinetic studies establish it as similar to 18F-FMISO. One possible disadvantage of 18F-EF5 is that the labeling chemistry is more complex than the simple nucleophilic displacement reactions used for the mono-fluorinated 2-nitroimidazoles (106).
Several single-photon–emitting radiolabels have been evaluated for labeling nitroimidazoles, including 123I and 99mTc. This research has been covered in recent reviews (43,45,57) and will not be described here as single-photon agents for imaging hypoxia have not found a clinical role.
In summary, it remains unclear whether any of the alternative 2-nitroimidazoles provides sufficient advantages over 18F-FMISO for a dominant role in clinical trials using nuclear imaging, given the substantial human experience with 18F-FMISO. The reality, perhaps unfortunate, is that once an imaging agent develops a clinical history with several hundred patients, a new agent has to be substantially superior to gain acceptance. Alternatively, it may be that several of the 2-nitroimidazoles with partition coefficients within a log unit of water will provide equivalent imaging results and could be combined in clinical trials. Perhaps we now have too many potential hypoxia tracers. We would argue that it is time to show whether imaging hypoxia is useful to the oncology community rather than developing more radiopharmaceuticals with marginally improved pharmacokinetic properties.
Cu-ATSM IMAGING OF HYPOXIA
An alternative PET agent for hypoxia is based on a metal complex of radioactive copper with ATSM, diacetyl-bis(N4-methylthiosemicarbazone) (44,107). Dithiosemicarbazones have antitumor properties that are enhanced when they are complexed with Cu(II). Because there are several advantageous imaging radionuclides of copper, this has led several laboratories to develop substituted ligands of dithiosemicarbazones as potential imaging agents (108). The first of these had 3 methyl substituents, PTSM, and was promoted as a blood flow agent (109). When another methyl was added to the diimine backbone, the Cu(II) complex was found to accumulate in hypoxic myocardium (107). The additional methyl had a minor effect on partition coefficient but a more substantial effect on reduction potential, E1/2, for Cu(II) → Cu(I). Testing in several tumor cell lines showed that uptake increased nearly 3x when cultured cells went from a PO2 of about 5 mm Hg down to less than 1 mm Hg, the radiobiologically significant range. Unfortunately, this hypoxic effect was not shown in all cell lines, and some cell lines showed no hypoxic selectivity at all (110,111). Furthermore, in a direct comparison involving Cu-ATSM, 18F-FMISO and pimonidazole in the SCCVII tumor model that has been widely used for studies of oxygenation effects, uptake of both 18F-FMISO and pimonidazole decreased as oxygenation increased, as would be expected for a hypoxia imaging agent, but uptake of Cu-ATSM increased under the same conditions (112).
These results led independent laboratories to study the mechanism of retention of Cu(II)-ATSM in some hypoxic cells. The approach involved a structure activity study of 13 64Cu-bis-thiosemicarbazones that differed only in number and location of alkyl or aryl substituents. The log P for this series ranged from 0.45 to 2.69 and E1/2 for Cu(II/I) ranged from −0.31 to −0.59 V with respect to a standard Ag electrode. The copper reduction reaction was monitored with a combination of cyclic voltammetry over a range of pH, optical spectrometry, and density function calculations. Alternative mechanistic arguments based on this work were critically reviewed by Vavera and Lewis (108). The simple mechanism is that reduction of Cu(II)-ATSM to Cu(I)-ATSM takes place continuously in both normoxic and hypoxic cells, but in the presence of O2 the copper is rapidly reoxidized to Cu(II)-ATSM which can freely diffuse in and out of cells. Cu(I)-ATSM is charged and trapped and may dissociate and transfer the radiocopper to other intracellular macromolecules. This is an attractive mechanism but fails to account for the variability with tumor type. Furthermore, it would not reconcile the paradoxical increase in Cu-ATSM uptake as PO2 increased after an episode of hypoxia. A recent review recommends additional studies be performed to deconvolute the copper reduction reaction from the basic coordination chemistry (108). Alternatively, it may be that changes in the thiol redox chemistry that occur as sequelae of hypoxia and HIF-1 activation are complicating the retention mechanism. Additional research elucidating the biochemical reasons why Cu-ATSM accumulates in tumors is urgently needed.
Preclinical studies with 62Cu-ATSM have shown that it is reduced and retained in hypoxic tissues, whereas it rapidly washes out of normoxic tissues (107). Cu-ATSM uptake is more rapid than 18F-FMISO uptake, and the reported hypoxic-to-normoxic ratio is greater (113–115). This is thought to reflect greater membrane permeability or more rapid blood clearance, although permeability is probably not the reason because early 18F-FMISO images accurately measure blood flow (70) and the image contrast of several 2-nitroimidazoles ranging over a wide value of partition coefficients is equivalent. Furthermore, the partition coefficients of Cu-ATSM and Cu-PTSM are essentially identical. The greater concern is that, because of its lipophilicity, the early uptake and washout of Cu-ATSM is probably influenced by regional blood flow, which is a major confounder with hypoxia.
Nevertheless, Cu-ATSM is finding a useful role in several clinical settings. In an initial study of 14 patients with biopsy-proven non–small cell lung cancer, 60Cu-ATSM uptake predicted response to therapy with radiation or chemotherapy (116). A more recent clinical study reported imaging of 15 patients with cancer of the uterine cervix who were imaged with 60Cu-ATSM (117). In this study, 481 MBq of 60Cu-ATSM were injected intravenously, followed by a 60-min dynamic imaging study. Uptake was assessed semiquantitatively by determining the tumor-to-muscle ratio from the summed 40- to 60-min data, as well as from a parametric map of a rate parameter. The rate and ratio data lead to the same conclusions, so the simpler ratio method was preferred and a threshold ratio of 3.5 was found to be an accurate cutoff for defining clinically significant hypoxia (118). In the cervical cancer study, Cox proportional hazards modeling demonstrated that hypoxia as determined by the PET images was a significant independent predictor of tumor recurrence. Four-year overall survival estimates were 75% for patients when the Cu-ATSM PET study was negative and 33% when it was positive. In this pilot study, the PET results were compared with several biomarkers linked with hypoxia: VEGF, COX2, EGFR, CA9, and apoptotic index, with the result that only CA9 and apoptosis were significant at P < 0.05 (117). VEGF was the poorest correlate of hypoxia. Although these patient numbers were small, the results clearly suggest further clinical evaluation is warranted, and some positive results should be expected within the next few years.
DIRECT MRI METHODS FOR MEASURING HYPOXIA
MRI methods for interrogating tumor oxygenation are attractive since they avoid the complication of short-lived radioactivity and MRI equipment is widely available. The most facile approach avoids the need for reporter molecules by imaging differences between O2Hb and deoxyhemoglobin (dHb), which is paramagnetic and increases the R2* (=1/T2*) relaxation rate of blood water. Changes in dHb are revealed by signal gain in T2*-weighted images, such as accompanying hyperoxic gas breathing, which increases vascular delivery of oxygen. BOLD MRI signal is related to vascular oxygenation and may allow direct estimates of PO2, for example, in the superior mesenteric vein or heart of children (119,120). However, the correlation becomes difficult for smaller blood vessels where partial-volume effects combine vessel and tissue in individual voxels (121). BOLD may also be confounded by flow effects, although appropriate pulse sequences can be used to minimize these. Howe et al. coined the term “FLOOD” to describe flow- and oxygen-dependent contrast using pulse sequences to decouple flow effects from the static effects of R2* (122,123). A correlation between BOLD and PO2 response in tumors has been reported (124). Baudelet and Gallez have rigorously investigated correlations between PO2 estimated using fiber optic probes and BOLD signal changes and have found general correlations, but a given BOLD response may reflect vastly different changes in PO2 (125). BOLD MRI has the advantage of both high spatial and temporal resolution and it can be repeated as needed. It is particularly attractive to patients, and IRBs rapidly accept oxygen breathing during MRI.
Historically, radiation biologists favored breathing carbogen (95% O2/5% CO2) over oxygen (100% O2) as an adjuvant intervention to reduce tumor hypoxia. Indeed, oxygen may alter hemodynamic parameters, potentially inducing hypotension with possible reduced delivery of oxygen to tumors with high interstitial pressure. Carbogen was used as part of the ARCON trial, which was reported to be successful (126,127). However, carbogen can cause respiratory distress in patients and is not always well tolerated. A lower CO2 content (2%) still modulates PO2 and with less patient discomfort (128). In our own studies, we generally found very similar results using oxygen or carbogen to modify tumor PO2 (129–133). Because of the differential effects of hyperoxic and hypercarbic gases, depending on vascular maturity (134), it appears that these gases deserve further evaluation with respect to BOLD studies in patients. To date, BOLD investigations have been largely exploratory in terms of methods of implementation. As a caution, they can be susceptible to motion artifacts that may be subtle (44).
BOLD studies have been reported in diverse human tumors (135–138). Rijpkema et al. used BOLD to evaluate patients during the ARCON trial for head and neck cancer and found significant changes in T2*-weighted MRI contrast accompanying hyperoxic gas breathing (128). No accompanying changes were observed by traditional T1-weighted Gd-DTPA dynamic contrast-enhanced MRI. At the San Antonio breast cancer meeting, preliminary data were presented for a group of 10 women being treated with chemotherapy for locally advanced breast cancer. There were a significantly different BOLD response to breathing oxygen before the course of chemotherapy for tumors of women with good therapeutic outcome versus those with poor response. Indeed, 3 women with complete pathologic response showed a signal change greater than 7%, whereas those with poor outcome showed less than 3% (139). It is not yet clear whether the differential response reflects perfusion or oxygenation, but traditional dynamic contrast-enhanced MRI failed to provide similar discrimination.
REPORTER MOLECULES FOR MR OXIMETRY
Oxygen-sensitive MR reporter molecules have also been developed, generally based on perfluorocarbons (PFCs). In this method, there is a linear relationship between R = 1/T1 of the 19F signal, and PO2 and calibration curves obtained in vitro are valid in vivo (140). Indeed, the extreme hydrophobicity of PFCs ensures that gases are highly soluble, providing molecular amplification of O2 concentration while ions are repelled and do not perturb the relation (141). Originally, perfluorocarbon emulsion blood substitutes were exploited, and Thomas et al. pioneered their application to assess vascular and tissue oxygenation (142,143). Although MR effects could be observed immediately after intravenous administration of PFCs, measurements were subject to flow artifacts (144). More generally, materials were administered intravenously or intraperitoneally and measurements were performed after complete vascular clearance. About 90% of the emulsion was sequestered in the reticuloendothelial system because of macrophage accumulation, and this approach allowed measurements of dynamic changes in PO2 in the liver, spleen, and even bone marrow (145–147). However, to achieve a sufficient signal-to-noise ratio for measurements in a reasonable time in tumors or the myocardium required administration of large quantities of PFC emulsion. This led to substantial hepatomegaly and splenomegaly and was unsatisfactory for general application (148,149). Moreover, PFC signal was often detected from the tumor periphery, corresponding to only the most well-perfused regions (150). These regions are typically the best oxygenated and thus the least important in terms of tumor response to oxygen-dependent therapy. Animal studies have been reported examining changes in PO2 in response to interventions such as breathing hyperoxic gas (129,131,133,151–154).
The traditional PFCs such as perfluorotributylamine have long-term tissue retention; half-life in liver may exceed 40 d. This has the advantage of allowing investigations of chronic long-term changes in PO2 over a period of weeks without readministering reporter molecule. Progressive hypoxia in tumors during growth over a period of 2 wk can be observed (150,155). Many blood substitute emulsions were based on materials with multiresonant spectra (e.g., perfluorotributylamine, perfluorotripropylamine, and perfluorooctyl bromide), which complicated the imaging experiment, usually leading to signal loss. The discovery of PFCs with a single signal improved signal-to-noise ratio. Mason et al. have favored hexafluorobenzene, C6F6 (156). Because of its high vapor pressure, C6F6 does not form effective emulsions but may be injected directly into tumors. Based on injections of 50 μL of pure C6F6, maps of PO2 may be obtained in rat tumors (Fig. 7) using echoplanar imaging in a method termed FREDOM (fluorocarbon relaxometry using echoplanar imaging for dynamic oxygen mapping) (15). The precision of measurements depends on the PO2 value, with better precision for the crucial low values and estimated precision of 1–3 mm Hg when PO2 is less than 5 mm Hg (15).
19F MRI oximetry of H460 human tumor xenograft implanted in nude rat. (A) Hexafluorobenzene (50 μL) was injected along multiple tracks directly into 0.57-cm3 tumor growing in thigh. FREDOM was used to acquire PO2 maps with in-plane resolution of 1.25 mm (5 mm thick) for 6.5 min each while anesthetized (1.5% isoflurane) rat breathed air and then after transition to breathing oxygen. Color maps show PO2 ranging from hypoxia to about 60 mm Hg at baseline, but with distinct spatial heterogeneity. (B) After switch to oxygen breathing for about 15 min, PO2 increased significantly, with many regions exceeding 60 mm Hg and hypoxia being essentially eliminated. Corresponding histograms are for air (C) and oxygen (D) breathing and show shift to right and essentially elimination of hypoxia in this responsive tumor. Cumulative frequency is overlaid on histograms.
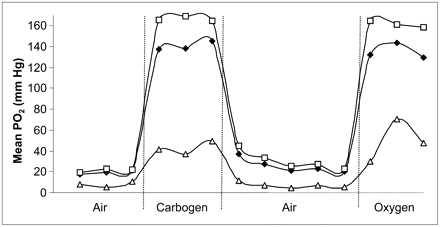
In developing any new assay, validation is crucial. Studies have shown that baseline PO2 measured using FREDOM is similar to that obtained using the Eppendorf histograph (157). Dynamic changes in PO2 response to interventions are commensurate with observations using polarographic oxygen electrodes (132) or the OxyLite and FOXY fiber optic probes (13,158). Moreover, relative hypoxia evaluated using pimonidazole and immunohistochemistry after tumor excision corresponded with baseline PO2 observed by FREDOM before sacrifice (159). The FREDOM approach has been applied to diverse rodent tumors and human tumor xenografts in mice (129,152,158–160). FREDOM has revealed distinct heterogeneity within and between tumors, both under baseline conditions and in response to interventions. Correlations have been shown in 2 Dunning prostate rat tumor lines between tumor PO2 at the time of irradiation and response to a single high dose of radiation. Furthermore, response to radiation can be modulated depending on the ability to alter hypoxia by breathing hyperoxic gas (Fig. 8) (160,161).
Dynamic oximetry showing regional response of tumor in Figure 7 to hyperoxic gas challenge. Sequential PO2 maps were acquired for 6.5 min each, as shown in Figure 7. Mean values are shown for region close to tumor periphery (□) and for tumor center (Δ), together with mean values for whole tumor (♦). As expected, tumor periphery had higher baseline PO2 and was far more responsive to hyperoxic gas breathing. Responses in both regions were rapid and reversible. It is apparent that global mean value was strongly biased by high values in peripheral region, emphasizing the importance of assessing regional variations.
Extensive toxicologic literature suggests that C6F6 could be used in patients once GMP is applied. Indeed, the LD50 has been found to exceed 25 g/kg (162–164). Perhaps the major obstacle to implementing 19F MRI in the clinic is the continued lack of availability of 19F NMR capabilities on human MRI systems, though it is increasingly becoming available in major clinical centers.
Given the current lack of availability of MRI systems capable of imaging 19F in humans, an analogous proton NMR approach (PISTOL [proton imaging of silanes for tissue oxygen levels]) has been proposed and tested in preliminary studies (165). Hexamethyldisiloxane (HMDSO) has properties closely matching C6F6 in terms of strong hydrophobicity, high oxygen solubility, low toxicity, ready availability, and inertness. Like C6F6, it exhibits a single NMR resonance whose spin lattice relaxation rate is sensitive to PO2. The chemical shift is about as far from water (4.7 ppm) and fat (1.3 ppm) as possible, occurring at about 0 ppm like the traditional proton NMR chemical shift standard tetramethylsilane. Water and fat suppression readily reveal HMDSO, which can be used to interrogate tissue PO2 after direct intratumoral injection. To date, results have been reported using NMR spectroscopy and imaging (165), and it remains to be evaluated with respect to therapeutic outcome. It is less volatile than C6F6, so HMDSO remains in tissues longer, which should assist with long-term studies of oxygenation. HMDSO may also allow effective emulsification to permit intravenous or intraperitoneal administration.
In summary, both the 19F MRI of C6F6 and the 1H MRI of HMDSO approaches exploit the physical interaction of oxygen dissolving in nanodroplets of reporter and reveal absolute PO2. Measurements are possible from anoxia to hyperbaric conditions, although the best precision is generally obtained below 10 mm Hg (166,167). This approach is conceptually different from the use of nitroimidazoles, which react chemically, leading to trapped adducts in hypoxic environments.
OTHER MR METHODS FOR IMAGING HYPOXIA
Three 2-nitroimidazoles chemically similar to those used for nuclear imaging have been labeled with multiple 19F atoms for MR detection. 19F NMR has a similar sensitivity to that of protons and has the advantage that there is essentiality no background signal. Thus, reporter molecules are readily identified. Introducing several equivalent atoms of 19F can enhance the signal-to-noise ratio still further. This approach to investigate hypoxia has been reported by several groups as reviewed previously (168–173). However, the achievable signal-to-noise ratio is typically disappointing, usually requiring long data acquisition times and generally precluding imaging. This method is predicated on formation of reactive intermediates that can react with diverse cellular components trapping the reporter molecule. In terms of nuclear medicine approaches, this provides specific uptake, which may be compared with normal tissues to reveal hypoxia. For NMR detection, multiple NMR-active species may be generated, each with a different chemical shift and, hence, a broad line or poor signal-to-noise ratio. Moreover, adducts formed as macromolecules have restricted rotational freedom, causing severe line broadening and loss of signal attributed to these effects (173). Recently, Koutcher et al. (174) successfully demonstrated low resolution 19F chemical shift imaging of trifluoroethoxy-MISO (TF-MISO) accumulation in rat breast and prostate tumors with a 1-h acquisition time after a dose of 75 mg/kg. Although the NMR approach avoids issues of radioactivity and limited shelf life, it requires administration of contrast agent at 400–1,600 mg/m2 (170), as opposed to less than 15 μg per patient for 18F-FMISO PET, and thus raises a concern about the toxicity and future clinical role of these reporter molecules.
Glycolysis, either aerobic or anaerobic, is often associated with tumors and is up-regulated by HIF-1 transcription. The end product of glycolysis is pyruvate and, in the presence of lactate dehydrogenase, LDH, which is also up-regulated by HIF-1, it catalyzes the quantitative formation of lactate, the same metabolite that can be measured by bioluminescence imaging. Mueller-Klieser has argued that determination of lactate in primary tumors may provide a useful classification for tumors that might lead to improved prognosis in clinical oncology (175), and a study involving patients with head and neck cancer showed that elevated tumor lactate predicted an increased risk of metastases (176). 1H MRI provides a good method for measuring relative regional lactate levels (177,178) as a consequence of hypoxia but is not a direct measure of tissue PO2.
ELECTRON SPIN RESONANCE
Reporter molecules have been developed for use with electron spin resonance (ESR or EPR), where the line width is highly sensitive to oxygen (179). Two primary approaches are used: direct injection of char crystals (180,181), phthalocyanine (182), or India ink (183) into tissue, most commonly tumors, although studies have also been reported of injection into brain, heart, and skeletal muscle; or intravenous infusion of water-soluble agents, which disseminate throughout the tumor vasculature (184,185). Measurements have traditionally used a spectroscopic approach to report PO2 at single locations only after direct implantation of reporter into a single tissue location. Nonetheless, significant data have been achieved demonstrating hypoxia and reoxygenation with respect to irradiation, and the importance of timing successive radiation doses to coincide with reoxygenation (186). Char particles may be stable in tissue for weeks to years, allowing measurements of chronic changes in tissues accompanying tumor growth (187). Bratasz recently presented a dramatic increase in molecular sensitivity to oxygen by dissolving perchlorotriphenylmethyl triester radical in hexafluorobenzene, exploiting the very high O2 solubility in HFB (188) as also exploited directly by 19F MRI (vide infra) (15). The intravenous approach is noninvasive, but reporter molecules may predominately distribute in the well-perfused vasculature, potentially biasing measurements toward the more well-oxygenated tumor regions. Progressive uptake and clearance of agents produce variable concentrations, and some agents degrade in tissue, requiring appropriate correction factors (185). Nonetheless, images of tumor oxygen distribution have been reported, including 3-dimensional representations (184), and Kuppusamy et al. have engineered high-speed electronics and algorithms for effective imaging. A recent innovation was predosing tumor cells with lithium octa-N-butoxy-naphtholcyanine, leading to uptake in culture. On subsequent implantation into mice, reporter molecules were retained, allowing PO2 measurements over a period of many days (189). Spin radicals may also be applied to a combined ESR/NMR approach (OMRI) exploiting the Overhauser enhancement in the tissue water proton MRI signal that occurs by polarization transfer from free radicals after electromagnetic irradiation (190). ESR approaches show promise, but the current lack of appropriate instrumentation prevents widespread implementation.
OPTICAL IMAGING METHODS FOR MEASURING HYPOXIA
Although in vivo optical imaging methods do not yet have a role in human studies of hypoxia, they have played an important role in evaluating hypoxia imaging methods and they continue to have a role in evaluation of biopsy specimens. Many biochemical pathways are under oxygen regulation and can provide an elegant window on hypoxia, for example, induction of HIF-1 and transcription of Glut-1 and other secondary responses such as increased production of VEGF (191). Intrinsic radiation sensitivity may also be assessed using the comet assay described later. These assays do not involve in vivo imaging; each requires biopsy of the tumor. Other markers potentially associated with hypoxia may be found in the plasma or urine and have been correlated with clinical outcome (192).
Several antibodies have been prepared that react with different nitroimidazole derivatives so that tissue specimens from patients who have been injected with gram levels of these drugs can be stained and viewed by immunohistochemical methods or by cell-sorting techniques. Pimonidazole is the most well known of these probes for animal imaging, and it is commercially available as Hydroxyprobe-1 (Natural Pharmacia International) (193). In one animal study, pimonidazole (120 mg/kg intravenously) was used to test the validity of CA9 immunohistochemistry as a biomarker of hypoxia. The spatial distribution and fractional staining of both markers was similar, but CA9 did not respond to carbogen reoxygenation, whereas pimonidazole staining decreased when PO2 returned to normal, establishing pimonidazole as the more accurate measure of hypoxia (91).
Pimonidazole binding has also been validated against the comet assay for measuring radiobiologic hypoxia (194). The comet assay is the name for a single-cell gel electrophoresis assay of single-strand breaks in DNA which Olive and Durand developed (195). Briefly, it involves exposing tumors to several grays of irradiation and then aspirating cells by fine needle and electrophoresing their alkaline lysate. DNA that has been broken by irradiation migrates faster, producing a comet-like tail that lengthens in direct proportion to the number of DNA breaks. This becomes a measure of radiobiologically significant hypoxia rather than simply a measure of PO2 as generated by a polarographic electrode. In a study involving a fairly homogeneous population of 58 patients with head and neck cancer, electrode and comet fine-needle measurements in the same metastatic neck nodes found no correlation between the biophysical measurement and the radiobiologic measurement (196). This observation challenges the validity of the Eppendorf PO2 histogram as a gold standard for clinically significant hypoxia.
Antibodies have been developed against the fluorinated 2-nitroimidazoles, EF3 and EF5. An immunohistochemical (IHC) assay for drug adducts of EF5 has been developed and applied to EMT6 spheroids to quantify radiobiologic hypoxia (197). The spheroids were incubated with EF5 in air-tight vessels with well-controlled PO2 and they were subjected to irradiation under the same conditions. Cryosections of the spheroids exposed to EF5 were stained with a fluorescent anti-EF5 antibody to identify regions of hypoxia. There was limited staining of the periphery and no staining in the necrotic center but intense staining of the interior, much as in the autoradiograph of Figure 4. Reoxygenated spheroids showed no fluorescent staining, nor did hypoxic cells not exposed to EF5. Cells dissociated from spheroids were assayed by flow cytometry for calculation of the percentage of stained cells and used to verify the accuracy of the imaging technique. Cells containing the highest level of EF5 binding were also the cells with the most radiation resistance.
IHC methods are particularly useful for in vitro studies, including assay of human biopsy specimens. In a study of 28 human brain tumors, a comparison of EF5 binding and the PO2 histogram revealed no statistical correlation between the 2 methods for detection of hypoxia although both methods indicated similar ranges for patients (198). Similar methods have been used to measure hypoxia in a series of patients with soft-tissue sarcomas. Patients were administered about 1.5 g of EF5 24–48 h before surgery, and biopsy specimens were stained as described previously. This pilot study supported the hypothesis that imaging hypoxia should provide an independent prognostic variable for outcome in high-grade sarcoma (199).
Tissue oxygenation can be measured directly using near-infrared oximetry to measure fractional oxygenation of Hb. This approach has been used intraoperatively in pigs to study regional myocardial oxygenation (200). The oxygenation levels were derived from spectroscopic images at 650 nm and the dHb levels at 1,050 nm. Light at these long wavelengths penetrates greater than 5 mm in tissues. Oxygenation in tissues correlated well with blood oxygen saturation levels. It is likely that this method will be more useful in the heart than in tumors because acute changes in O2 cause stress in the heart whereas tumors adapt to chronic hypoxia. This approach may be clinically useful in open-chest surgery. The nitroimidazoles have not proven useful for imaging myocardial hypoxia (70,201,202).
In a related method, sensors using quenching of the near-infrared fluorescent or phosphorescent lifetime of inorganic crystals by O2 have been used for imaging PO2 in cutaneous melanoma compared with adjacent skin that was clinically normal (203). The average difference between melanoma and normal tissue was 10 mm Hg, and the lowest average tumor PO2 was 8 mm Hg, higher than in many solid tumors. It is not likely that this approach will have widespread clinical value, but it should find a role in small-animal studies and is finding an important role in cell biology (204) as new and more photostable chromophores are developed. The oxygen-quenching effect is rapidly reversible so that this assay is useful for observing rapid changes in oxygenation status, including reoxygenation. Furthermore, optical probes at different wavelengths can be used in the same cells to study different biochemical processes simultaneously, a feature that is particularly advantageous for optical systems and cannot be achieved with the other hypoxia imaging systems considered here.
Alternatively, surrogate markers of hypoxia have been measured. Bioluminescent imaging has been applied to flash-frozen tissue samples to detect, at the microscopic level, various characteristics of a tumor microenvironment, including metabolites such as glucose, ATP, and lactate. Walenta has provided a useful review of this technique (205). For example, bioluminescent imaging of lactate in specimens from patients with head and neck and uterine cervical cancer has established that a high level of lactate from glycolysis is associated with an increased rate of metastasis and poor outcome (206). Bioluminescent imaging provides an important tool for detailed examination of human biopsy material that will support translation of human observations to more detailed bench studies with small animals, cells, and tumor spheroids. For example, it is complementary to autoradiographic studies using PET radiopharmaceuticals as one way to validate the mechanistic interpretation of PET images.
Some elegant methods have been developed to directly measure HIF-1 activity by the introduction of transgenes with hypoxic response elements as promoter sequences coupled to reporter genes such as luciferase (207,208) or green fluorescent protein, GFP (209). A luciferase reporter gene under regulation of an HIF1-dependent promoter has been developed (210) for in vivo bioluminescent imaging. It is stable, expresses 100-fold increased luciferase in response to hypoxia, and has been used to evaluate the efficacy of a hypoxia-directed therapy in animals. Bioluminescence accompanying luciferase requires ATP and O2, but reports suggest that even under exceedingly low PO2, sufficient oxygen remains to reveal hypoxia. Another experiment used a retroviral vector with an HIF-1–inducible reporter gene for GFP (211). GFP synthesis is another energetic process that could be hindered under hypoxic conditions. These imaging tools are useful for studying the biology of hypoxia and mechanisms of response to experimental therapy but are unlikely to have a role in human imaging.
Imaging techniques based on optical contrast between O2Hb and dHb can be combined with ultrasound detectors in a method called photoacoustic tomography, PAT. This technique overcomes the resolution limitations imposed by scattered photons in purely optical imaging. PAT retains intrinsic optical contrast but it uses an ultrasound (US) transducer to detect the resulting photoacoustic waves. A laser that emits visible light at a wavelength where O2Hb and dHb absorb is pulsed for a short interval. Optical absorption in the laser-irradiation region generates photoacoustic waves proportional to the distribution of absorbing materials. The optically excited molecules produce a photoacoustic wave that can be detected by a very sensitive wide-bandwidth US transducer. This photoacoustic signal has the high spatial resolution and depth penetration characteristic of US, making it a practical method for human studies. PAT is a new technology but one with considerable promise. Although its role in human patients has not been evaluated, some early studies in animals show considerable promise (212–214).
CLINICAL ROLE OF HYPOXIA IMAGING
Identifying hypoxic tissue has therapeutic implications for multiple disease states including stroke, myocardial ischemia, and diabetes, as well as tumors (78). The selectivity of nitroimidazoles for hypoxic regions has been demonstrated in rat myocytes (69), the gerbil stroke model (215,216), pig and rat liver (217–219), and dog myocardium (201,202). In ischemic stroke, 18F-FMISO was able to identify the areas of brain tissue to which a stroke extended (220,221). 18F-FMISO has been evaluated for identifying chronic myocardial hypoxia as an alternative marker for viability (52,222). 18F-FMISO distribution in the heart and other organs principally reflected the blood–tissue partition coefficient, except in liver and kidney, where the whole organ concentration was consistently higher than in blood.
The growing body of literature showing that hypoxia identified by imaging is predictive of poor survival in numerous cancer settings was reviewed along with each of the imaging agents. The next phase of studies evaluating the predictive value of hypoxia imaging in tumors needs to provide convincing data on the extent to which it is an independent predictive marker, in view of the many assays available to the oncologist. Furthermore, these trials needs to consider all therapeutic strategies, not just radiation therapy, in view of the mounting evidence that HIF-1 signals for upregulation of multiple factors that increase the survivability and metastatic spread of cancer cells (22,35,36,40,43).
The initial motivation for developing hypoxia imaging was the cure limitation imposed by hypoxia in response to radiation therapy. When all patients were treated with various strategies designed to overcome the cure limitations of hypoxia, the results were disappointing (23,24). However, several groups have suggested that if only those patients with substantial levels of hypoxia were treated with escalating doses of ionizing radiation, a beneficial improvement in outcome might be achieved. Although it is unrealistic to increase the dose 3-fold, the value of the OER, a modest increase of 10 or 20 Gy might achieve significantly better local control. We are analyzing our 18F-FMISO results in HNSCC (91) to test the hypothesis that primary regrowth occurs more frequently in sites that are 18F-FMISO–positive and might potentially be treated with higher doses of radiation using image-guided treatment planning (79,223).
Because hypoxia is a common although not universal attribute of the tumor phenotype, efforts have been directed toward exploiting this characteristic in therapy. The hypoxic tumor phenotype could be turned to therapeutic advantage through drugs that are activated to cytotoxins only in the absence of normal PO2. The first of these drugs to be tested in the clinic was tirapazamine (TPZ), originally referred to as SR4233 (224). The TPZ radical formed by electrons from 1 e− reductases such as the cytochrome P450 reductases will abstract hydrogen atoms from DNA, producing both single- and double-strand breaks. Under aerobic conditions, O2 can remove the additional electron before it gets close to a critical DNA target. TPZ and ionizing radiation act as complementary cytotoxins in that each one kills cells that are resistant to the other. TPZ also enhances the effectiveness of cisplatin, CIS (225). In a clinical trial of patients with HNSCC, those with hypoxic tumors (18F-FMISO–positive) who received TPZ/CIS fared significantly better than the CIS/FU patients with hypoxic tumors with respect to the risk of locoregional failure (81,226). This outcome supports the efficacy of TPZ in the presence of hypoxic disease. Although normal-tissue toxicity has now stopped the clinical trials of TPZ, clinical trials of newer cytotoxins such as PR-104A have started. It is a soluble phosphate ester that is converted in vivo to the corresponding alcohol and then activated under hypoxic conditions to a cytotoxic nitrogen mustard prodrug (227). It will be important to assess whether these new agents reduce the quantity and distribution of hypoxic disease, something that should be feasible with hypoxia imaging.
CONCLUSION
Thus, molecular imaging of hypoxia has 2 clinically important roles, selecting a cohort of patients who might respond better to treatments designed to overcome the cure limitations of hypoxia and documenting through serial imaging that the treatment strategy reduced the extent of hypoxic disease. Molecular imaging has provided the oncology community with several options to choose from in assessing regional hypoxia. It takes molecular imaging beyond the role of tumor detection to the role of tumor characterization in support of personalized medicine.
Acknowledgments
We gratefully acknowledge support from P01 CA42045 and S10 RR17229 from the National Institutes of Health; and support from the Simmons Cancer Center, Southwestern Cancer Imaging Program (SW-SAIRP U24 CA126608); and NIH BTRP facility P41-RR02584. We are grateful to Jesús Pacheco-Torres and Drs. Dawen Zhao, Vikram Kodibagkar, and Debu Saha for allowing us to present the unpublished observations shown in Fig. 7.
Footnotes
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.
- 9.
- 10.↵
- 11.↵
- 12.
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.
- 85.↵
- 86.
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.
- 137.
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵
- 161.↵
- 162.↵
- 163.
- 164.↵
- 165.↵
- 166.↵
- 167.↵
- 168.↵
- 169.
- 170.↵
- 171.
- 172.
- 173.↵
- 174.↵
- 175.↵
- 176.↵
- 177.↵
- 178.↵
- 179.↵
- 180.↵
- 181.↵
- 182.↵
- 183.↵
- 184.↵
- 185.↵
- 186.↵
- 187.↵
- 188.↵
- 189.↵
- 190.↵
- 191.↵
- 192.↵
- 193.↵
- 194.↵
- 195.↵
- 196.↵
- 197.↵
- 198.↵
- 199.↵
- 200.↵
- 201.↵
- 202.↵
- 203.↵
- 204.↵
- 205.↵
- 206.↵
- 207.↵
- 208.↵
- 209.↵
- 210.↵
- 211.↵
- 212.↵
- 213.
- 214.↵
- 215.↵
- 216.↵
- 217.↵
- 218.
- 219.↵
- 220.↵
- 221.↵
- 222.↵
- 223.↵
- 224.↵
- 225.↵
- 226.↵
- 227.↵
- Received for publication October 26, 2007.
- Accepted for publication March 11, 2008.