Abstract
Pre- and postsynaptic cardiac sympathetic function is altered in ischemic congestive heart failure (CHF). Whether there is a presynaptic-to-postsynaptic mismatch or whether mismatch is related to adverse cardiac events is unknown. Methods: In 13 patients with ischemic CHF and 25 aged-matched healthy volunteers, presynaptic function was measured by PET of 11C-meta-hydroxyephedrine (11C-mHED), a norepinephrine (NE) analog. Postsynaptic function, β-adrenergic receptor (BAR) density (B′max), was measured by imaging 11C-CGP12177. Myocardial blood flow (MBF) was measured by imaging 15O-water. Each heart was analyzed both globally and regionally, excluding infarcted regions, and a mismatch score, defined as the ratio of B′max to NE uptake (PSnt), was used to indicate mismatch of post- and presynaptic function. Results: Global and regional MBF was not different between CHF and healthy subjects. The global measure of PSnt was lower in CHF (0.32 ± 0.34) than that in healthy subjects (0.81 ± 0.33, P < 0.0001) and in all 12 regions. Global B′max tended to be lower in CHF than that in healthy subjects (10.0 ± 6.4 pmol/mL vs. 13.4 ± 4.2, P = 0.056) and in all 12 regions. The global mismatch score (B′max:PSnt) in CHF patients was significantly greater than that in healthy subjects (50.3 ± 50.7 vs. 19.3 ± 9.7, P = 0.005) and also greater in 11 of 12 regions. After 1.5 y of follow-up, 4 individuals had an adverse outcome (CHF death, new or recurrent sudden death, or progressive CHF leading to transplantation). Three of the 4 had mismatch scores > 3 times that of the healthy subjects or the CHF patients without an adverse outcome. Conclusion: Mismatch between pre- and postsynaptic left ventricular sympathetic function is present in patients with severe CHF and may be more marked in those with adverse outcomes.
Sympathetic function is abnormal in congestive heart failure (CHF) patients, who demonstrate increased sympathetic nerve activity, norepinephrine (NE) plasma levels, cardiac NE spillover, and depleted cardiac NE stores (1–4). This increased cardiac adrenergic drive may involve increased neuronal NE release, decreased efficiency of NE reuptake by the NE transporter (NET-1), or reduced vesicular storage (5–7). In animals, regional heterogeneity of sympathetic nervous system (SNS) function and reenervation has been observed in myocardial infarct borders (8–10). Such regional heterogeneities may cause or exacerbate arrhythmias.
Regional heterogeneity of 11C-meta-hydroxyephedrine (11C-mHED) uptake and retention has been demonstrated in patients with coronary artery disease (CAD) and ventricular tachycardia/fibrillation and hypothesized to increase risk for sudden cardiac death (SCD) (11). Heterogeneity of 11C-mHED was also observed in patients with left ventricular (LV) dysfunction (12–14). In nonischemic dilated cardiomyopathy, marked regional variation in 11C-mHED uptake correlated with variations in tissue NE reuptake density from explanted, diseased hearts (15).
β-Adrenergic receptor (BAR) density is decreased in both ischemic and nonischemic CHF compared with age-matched healthy subjects (2,3,16). PET has shown that global BAR density is lower in patients with CHF from idiopathic dilated cardiomyopathy (IDCM) compared with that of healthy subjects (17).
In humans with arrhythmogenic right ventricular dysplasia, which predisposes to fatal arrhythmias, there is a marked (∼40%) reduction of postsynaptic β-receptor density and a lesser decrease in presynaptic function (18). This possible imbalance in pre- and postsynaptic function can be termed a mismatch and is likely to also be present in CHF. The purpose of this study was to determine the extent and magnitude of regional mismatch between pre- and postsynaptic function in patients with ischemic CHF in comparison with that of age-matched healthy subjects and to relate mismatch to adverse events during follow-up.
MATERIALS AND METHODS
The 2 populations studied were patients with CAD and CHF from depressed LV function and healthy older volunteers. Twenty-five healthy sedentary volunteers (mean age ± SD, 72.0 ± 3.6 y; range, 65–79 y) were studied. The healthy volunteers were screened to exclude current smoking, hypertension, chronic medication use, or any cardiovascular or pulmonary disease. All had unremarkable blood counts and chemistries, including cholesterol and thyroid-stimulating hormone, urinalysis, normal 2-dimensional and Doppler echocardiograms for age, and normal maximal postexercise SPECT 99mTc-sestamibi images. Thirteen were female (mean age, 71.3 ± 3.6 y) and 12 were male (age, 72.2 ± 3.8 y). All females were receiving hormone replacement therapy.
The CHF subjects (Table 1) consisted of 13 male patients (mean age ± SD, 69.8 ± 5.6 y; range, 60–75 y; P = not significant [NS] vs. healthy subjects) with CAD and an LV ejection fraction (EF) ≤ 45% by catheterization or 2-dimensional echocardiography (mean EF, 32% ± 9%). Seven patients had implanted defibrillators because of a previous episode of symptomatic, sustained ventricular tachycardia or ventricular fibrillation > 6 wk before the PET study. Eight subjects were on chronic β-blocker therapy (mean dose, 96 ± 68 mg metoprolol daily), which was withheld for >24 h before imaging. Other medications (Table 1) were taken as usual. No patient was taking medications known to directly affect presynaptic sympathetic function.
Clinical Characteristics of CHF Subjects
This study was approved by the University of Washington Human Subjects, Radioactive Drug Research, and Radiation Safety Committees. All subjects gave written informed consent.
Study Protocol
Radiotracer Synthesis.
15O-Water (19), 11C-mHED (20), and the BAR antagonist (S)-(−)-11C-CGP12177 ((S)-4-(3′-t-butylamino-2′-hydroxypropoxy)-benzimidazol-2-11C-one), 11C-CGP12177 (11C-CGP) (21–23), were synthesized according to published methods.
Metabolites of 11C-mHED were measured as reported (24). (S)-(−)-CGP is not metabolized in humans (25). The amount of nonradioactive material in the 11C-CGP and the 11C-mHED injectates was measured using high-performance liquid chromatography (HPLC) with mass spectrometry (MS) (ES+) detection (Waters 2190 and Micromass ZMD) (14).
Imaging Protocol.
Heart rate and blood pressure were monitored continuously and recorded each minute from 5 min before to 15 min after each radiotracer injection. The subjects were positioned in the tomograph (Advance; GE Healthcare) using 1-min transmission scans to localize the heart (35 planes, 15 cm). Twenty-minute transmission scans were acquired using a rotating 68Ge rod source. The imaging protocol (Fig. 1) consisted of sequential injections of high-specific-activity (SA) (3.7 MBq/kg average, 2.76 × 107 mBq/mmol) 11C-CGP followed approximately 20 min later by injection of 3.7 MBq/kg low SA (average, 2.23 × 106 MBq/mmol) 11C-CGP as described previously (26) followed by 15O-water and 11C-mHED. Activity (mCi) of each injectate was measured using a calibrated ion chamber.
Time line for radiotracer injection (arrows) and PET image acquisition. Attn = transmission image acquisition; CGP1 = high-specific-activity 11C-CGP; CGP2 = low-specific-activity 11C-CGP; H2O = 15O-water.
For the first CGP injection, dynamic PET images (60 s × 1, 5 s × 2, 10 s × 6, 15 s × 6, 30 s × 4, 60 s × 15) were acquired for 21 min (Fig. 1), starting 1 min before the first CGP injection (CGP1). Low SA CGP (CGP2) was injected ∼25 min after the CGP1 injection. The CGP1 dynamic image sequence was repeated and continued for 45 min by adding 5-min frames. Myocardial blood flow (MBF) was measured with a bolus injection of 18.5 MBq/kg of 15O-water. Dynamic PET images were acquired for 5 min. After 15 min for 15O decay, 11C-mHED (7.4 MBq/kg) was infused with the dynamic PET image acquisition sequence as for CGP2. The 11C-CGP and 11C-mHED were injected over 1 min. Subjects were in the tomograph for the entire imaging protocol. Heparinized plasma samples from ∼5, 10, 20, 30, and 40 min after 11C-mHED injection were analyzed for 11C-mHED and metabolites (24).
The dynamic PET image sets were reconstructed, reoriented into short-axis cardiac projections, and analyzed. Images were decay-corrected to the time of each radiotracer injection, except for the CGP2 images, which were corrected to the start of the CGP1 injection. Myocardial and left atrial (LA) regions of interest (ROIs) were placed on static short-axis images from each set. These ROIs were applied to the dynamic images and time–activity curves generated for 3 middle LA planes and 96–128 myocardial ROIs per heart (12–16 slices starting at the apex depending on heart size and with 8 sectors per slice). Three LA planes were averaged to provide a single LA time–activity curve for each image set, which is used as the input function for the respective model analysis described below. The 3 most apical LV planes were excluded from analysis to avoid any partial-volume effect from this region. After quantitative analysis of the remaining individual ROIs, the slice data were averaged to give 3 cross-section slices labeled apical, mid, and basal. Then, 2 adjacent sector's ROIs were averaged to give 4 regions per slice: anterior, lateral, inferior, and septal. This resulted in 12 LV ROIs and a global ROI (average of the 12) per subject for each of the 4 radiopharmaceutical injections.
The 15O-water time–activity curves (cpm/pixel) from the LA and from each of the individual LV ROIs were modeled to obtain MBF (27). The 11C-CGP time–activity curve data were converted to pmol/mCi at the time of first injection based on the SA of the injectate. Some of the CHF patients had prior myocardial infarctions (MIs) with thin walls. To minimize MI partial-volume effects, we excluded regions in which the resting MBF was <0.16 mL/min/mL from analysis. This is the lowest value for any single ROI in the 25 healthy subjects and 3.5 SD below the normal mean MBF. The low MBF ROIs were excluded before the summing of slices that gave the final 12 ROIs used for comparisons between pre- and postsynaptic function. Only 5% of 1,288 MBF ROIs were excluded from the CHF patients. This resulted in an average of 12.7 ± 8.7 excluded ROIs from 6 of 13 patients.
Data Analysis
11C-mHED metabolites, expressed as the fraction of the whole-blood activity, contribute to the blood PET signal (11). These fractions were curve fit to a rising time-dependent exponential function of the form, f(t) = y0 + (a*(1 − exp(−b*t))) using SigmaPlot (SYSTAT Software). Using these derived values, a metabolite-corrected LA cavity time–activity curve was generated by multiplying the LA cavity activity by the function (1 − f(t)). Representative metabolite-corrected and uncorrected LA time–activity curves and a LV ROI time–activity curve from a healthy subject are shown in Figure 2.
Decay-corrected 11C-mHED time–activity curves from myocardial and metabolite left atrial cavity (LA Cav) ROIs in CHF patient with our model fit to myocardial time–activity curves. (Left) Location of ROI 8 (arrowhead), a visually “normal” region, and the corresponding myocardial time–activity curve. (Right) Location of ROI 3, an abnormal mHED accumulation, is shown. ROIs are bounded by inside and outside arcs within each of 8 radial lines. Parameter estimates are given for PSnt, PSves (11C-mHED release by vesicles), V′nt (virtual volume of nerve terminal), and Gseq (rate vesicular storage of mHED). MBF and the retention fraction (RF) of 11C-mHED for the 2 ROIs are also shown.
11C-mHED kinetics were modeled by blood tissue exchange as previously described and uses the MBF value from the 15O-water study to provide the flow parameter in the model (14,28). This model expresses 11C-mHED kinetics in terms of the permeability·surface area product (PSnt, mL/min/mL tissue) for 11C-mHED uptake into the nerve terminal from the interstitial space (ISF) through the NET-1 process and PSves for release of 11C-mHED back into the ISF through exocytosis. The neuronal storage of 11C-mHED is expressed as a virtual volume (V′nt, mL/mL tissue) and the rate of vesicular storage of mHED is expressed as Gseq (mL/min/mL). Transport rates and volumes are expressed as milliliter of tissue without conversion for any tissue density. The parameter PSnt most closely represents 11C-mHED uptake into the nerve by NET-1 and is the process most likely to be affected by myocardial ischemia or injury. BAR density (B′max) was estimated for each myocardial ROI using the Delforge method (26,29).
A mismatch score, defined as the ratio of B′max to PSnt, is used to indicate mismatch of post- and pre-SNS function.
Statistics
Hemodynamic measurements, MBF, and SNS measures, B′max, PSnt, PSves, V′nt, and Gseq were compared between the healthy subjects and the CHF patients using a 2-tailed, unpaired Student t test. Hemodynamic measurements before and after radiotracer injections were compared using paired t tests. Statistical analyses used SPSS.
RESULTS
Hemodynamics
Heart rate and blood pressure before and during imaging are presented in Table 2. Five 1-min preinjection recordings were averaged as baseline for each injection. The greatest change in each measure within 0–15 min after injection was compared with the baseline data (Table 2). Compared with preinjection values, postinjection hemodynamics after CGP injection in both healthy subjects and CHF patients were statistically different but not clinically significant.
Hemodynamics Before and After Radiotracer Injection
Images
After excluding myocardial regions with MBF < 0.16 mL/min/mL, the global average resting MBF was 0.76 ± 0.20 mL/min/mL in the CHF patients vs. 0.78 ± 0.15 in the healthy subjects (P = NS; Fig. 3). MBF did not significantly differ for any of the 12 regions between healthy subjects and CHF patients, consistent with exclusion of infarcted tissue from the analyses.
Box-and-whisker plots for global values for MBF (left) and B′max (right) for healthy subjects (normal) and CHF patients. Box represents 25%–75% of data; whiskers represent 5%–95%; heavy and thin solid lines are mean and median values, respectively.
Representative short-axis tomographic slices of 11C-mHED and 11C-CGP images (Fig. 4) are shown for a patient with an LV EF of 0.35. For 11C-mHED, only the anterior–septal regions appear to have normal uptake. In contrast, the 11C-CGP images show uptake in all regions except for the site of a prior inferior MI.
Short-axis PET images of 11C-mHED (35- to 45-min sum) and 11C-CGP (10- to 20-min sum from injection 1) in CHF patient. Apical slices are at upper left and basal slices are at lower right of each panel. Arrows indicate extensive mismatch between 11C-mHED and 11C-CGP.
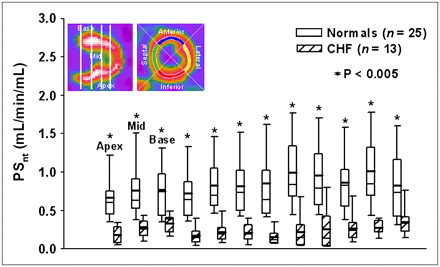
Global PSnt, 11C-mHED nerve uptake by NET-1, was significantly lower for CHF patients (0.32 ± 0.34 mL/min/mL) than that for healthy subjects (0.81 ± 0.33, P = 0.0001). PSnt was lower and varied more between the 12 regions of the CHF patients compared with that of healthy subjects (Fig. 5). PSnt did not differ between subjects who did not take β-blockers and those who had them discontinued for 24 h before imaging.
Box-and-whisker plots for regional NE transport (PSnt) for 12 LV regions per subject. Locations of apical, middle, and basal slices lie between the white vertical bars on the long-axis image at upper left. Locations of large sectors—anterior, lateral, inferior, septal—are shown on short-axis image. Normals = healthy subjects.
Global B′max was 22% lower in the CHF patients compared with that of healthy subjects (global, 10.0 ± 6.4 vs. 13.4 ± 4.2 pmol/mL), which was of borderline statistical significance (P = 0.056) (Fig. 3). Regional B′max also tended to be lower (data not shown). Global B′max did not differ between subjects who did not take β-blockers and those who had them discontinued for 24 h before imaging. This was expected, as the average plasma half-life of metoprolol is 3.5 h.
The mismatch score was defined as the ratio of B′max to PSnt and is used to indicate mismatch of post- and pre-SNS function. The global average mismatch score was significantly greater and more variable in the CHF patients than that in the healthy subjects (50.3 ± 50.7 vs. 19.3 ± 9.7, P = 0.005). Regional mismatch scores for all 12 regions were significantly higher in the CHF patients than those in the healthy subjects (all P < 0.05; Fig. 6).
Box-and-whisker plots of mismatch score, which is the postsynaptic-to-presynaptic ratio (B′max:PSnt) for the same 12 LV regions as in Figure 5. Normals = healthy subjects.
The parameters PSves and Gseq did not differ between healthy subjects and CHF patients. The difference for mean V′nt between the 2 populations was significant, but with large SD, and, thus, not physiologically meaningful. The parameters V′nt, Gseq, and PSves are not independent. To account for codependence, we examined the 3 parameters as a “lumped” parameter, LP: There was a nonsignificant trend for the global and regional LPs to be lower in CHF patients than that in healthy subjects. However, if we omitted either the LP or the 3 individual parameters, the model would not fit the myocardial ROI data, indicating that these parameters, though less physiologically sensitive than PSnt, are necessary for the model. Because the CHF population was all male, for all parameters, we then compared the 13 CHF patients to just the 12 healthy males. The results were unchanged.
There was a nonsignificant trend for the global and regional LPs to be lower in CHF patients than that in healthy subjects. However, if we omitted either the LP or the 3 individual parameters, the model would not fit the myocardial ROI data, indicating that these parameters, though less physiologically sensitive than PSnt, are necessary for the model. Because the CHF population was all male, for all parameters, we then compared the 13 CHF patients to just the 12 healthy males. The results were unchanged.
Follow-up
All CHF patients were followed for at least 18 mo. Four patients had an adverse event. One underwent cardiac transplantation for worsening CHF at 3 mo after the PET study. One died of progressive CHF 6 mo after PET. One patient without an implantable cardioverter-defibrillator (ICD), who previously had an episode of ventricular tachycardia, suffered a recurrent episode for which he received an ICD. One patient without a prior history of arrhythmia but with an EF of 0.35 had an episode of nonresuscitable sudden death 6 wk after PET.
The mean and range of regional B′max-to-PSnt mismatch scores within each individual subject were compared in the healthy subjects and in subjects with and without adverse cardiac events. All 25 healthy subjects had close matching of pre- and postsynaptic function and none had an adverse event. In contrast, 3 of 4 patients with an adverse event had a mean mismatch score > 6 SD above the mean of the healthy subjects (Fig. 7). Of the CHF patients with a mean mismatch score greater than the upper limit of normal (2× SD), 43% had an adverse event. The same comparisons done using only the healthy males did not change the findings. Examination of individual heterogeneity of presynaptic function, PSnt, or postsynaptic B′max did not correlate with any of the individual patients with subsequent adverse events.
B′max:PSnt from the 12 ROIs per individual subject are displayed as box-and-whisker plots (mean = heavy solid line). The 5%–95% whiskers indicate within-subject B′max:PSnt heterogeneity. Horizontal dotted line indicates 2 SD above the mean B′max:PSnt of healthy subjects (normals). Patients with an adverse outcome at 1.5 y of follow-up are indicated by the symbols shown.
DISCUSSION
To our knowledge, this study is the first to demonstrate significant SNS mismatch between pre- and postsynaptic function in the same myocardial regions in patients with moderate-to-severe CHF. This study is also the first to demonstrate the potential of mismatch as a marker of adverse outcome in patients with CHF.
Our findings of decreased presynaptic function, measured by 11C-mHED imaging, in patients with ischemic CHF are consistent with previous reports (13,30,31). Global B′max was 22% lower in our CHF patients than that in the healthy subjects and was similarly decreased for all regions. Although not statistically significant, it is consistent with the 9% total (14% for β1) BAR density decrease from in vitro assays of biopsy samples of patients with ischemic CHF compared with age-matched healthy subjects (16). In another study, global PET BAR density was decreased in patients with CHF from idiopathic IDCM and correlated with biopsy samples in the same subjects (17).
A measure than can identify individuals rather than populations at high risk for future cardiac events is needed. Few studies have evaluated both pre- and postsynaptic function in the same individuals, and none have been previously done in a single day. Six patients with hypertrophic cardiomyopathy and normal LV function had decreased average LV 11C-mHED uptake (53%) and BAR density (28%) compared with that of healthy subjects (32). These authors postulated that decreased 11C-mHED uptake was due to impaired NET-1, which increased local catecholamines and downregulated BAR. Wichter et al. observed a 17% reduction in 11C-mHED uptake but a 42% reduction in average LV BAR density in 8 patients with arrhythmogenic RV cardiomyopathy compared with that of healthy subjects, suggesting considerable mismatch in this population (18). Ungerer et al. sampled tissue from explanted IDCM human hearts (n = 9) and found no correlation between pre- and postsynaptic function (BAR density); the noninvasive measure of 11C-mHED uptake did correlate with the in vitro measure of NET-1 (r = 0.65) (15). However, the tissue preparation they used measured total BAR density, not the surface-active receptors measured by 11C-CGP12177 imaging. They also found an inverse relationship (r = −0.61) between BAR kinase1 (βARK-1) and NET-1 binding and postulated that regional βARK-1 has a greater response to presynaptic stimulation than does BAR density. The suggestion from these prior studies is that both pre- and postsynaptic function is important but varies widely in disease.
Our results show that, both globally and regionally, BAR density and 11C-mHED uptake are tightly matched in healthy subjects, whereas patients with ischemic CHF have much greater global and regional mismatch by B′max:PSnt (Fig. 6). Increased BAR relative to partially functioning presynaptic sympathetic nerves could increase the arrhythmogenic potential by increasing the sensitivity of myocardial regions to increased NE levels, leading to local increases in the adenylyl-cyclase pathway and increased spontaneous depolarization or regional changes in sodium or potassium channel activity affecting repolarization.
The mechanism for the observed mismatch is unknown. The presynaptic component of the cardiac sympathetic nervous system is sensitive to ischemic insult (9,33). In patients with ischemic CHF, we anticipate decreased or lost presynaptic innervation or abnormal NET-1 or NE storage or release. Sympathetic signaling in such regions would be more dependent on circulating catecholamines, which are probably lower than those in a normally functioning myoneural junction (3). This decrease could lead to BAR upregulation. BARs are not upregulated in the infarct but may be at the margins (3,34,35). We excluded the most severely infarcted regions from analysis to minimize the partial-volume and spillover effect that would be present in the center of the infarct zones and likely to produce model estimates of both NE transport and BAR density when none exists. Outside the central infarct zone, impaired reuptake because of partial sympathetic nerve dysfunction could lead to excess local NE with a compensatory decrease in BAR density. Our data are consistent with observations of decreased, but not absent, presynaptic function in the periinfarct region combined with a small decrease in postsynaptic function (16,36).
When studying mismatch, and mismatch heterogeneity, it is advantageous to study the smallest possible coregistered regions of sequential images. Our PET method approaches this goal. Regional mismatch (Fig. 6) is more striking than the global mismatch. In this small number of patients, the magnitude of mismatch was greatest in the lateral and inferior regions without any gradient between apex and base.
Not only do CHF patients have greater mismatch between presynaptic function and postsynaptic BAR density than that of healthy subjects, but our results suggest that individuals with the largest number of mismatch regions and the greatest magnitude of heterogeneity may be predisposed to an adverse event (Fig. 7). These findings are new. Previous studies of presynaptic function alone, using either methoxyisobutylisonitrile (MIBG) uptake and clearance or 11C-mHED retention fraction (RF) in CHF populations have suggested that those with the fastest MIBG washout or an RF < 0.18 have a higher SCD or transplant event rate (37,38). In our population, RF (not shown) and heterogeneity of presynaptic function (by RF or PSnt) were not predictive. All but 1 of our subjects had an RF < 0.18 and that subject did not experience an event. The difference between our findings and others for presynaptic function may relate to patient populations or numbers. The previous studies contained a mixture of ischemic and nonischemic cardiomyopathies. Although the number of CHF patients in our study is too small to draw a conclusion, the mismatch data suggest that measures of heterogeneity of mismatch may be sensitive indicators of an individual's risk. Studies of a larger number of CHF patients, categorized as to etiology of CHF and with longer follow-up will be required to establish the prognostic value of mismatch of B′max:PSnt.
Our study demonstrates the feasibility of performing PET studies of pre- and postsynaptic sympathetic function using 11C radiotracers and MBF (15O-water) sequentially within ∼3.5 h. Performing all imaging in the same session without patient movement minimizes misalignment of cardiac regions on the images and differences in systemic catecholamines that might influence sympathetic function. Our study also demonstrates that radiopharmaceuticals used to measure pre- and postsynaptic sympathetic function with PET do not cause clinically significant hemodynamic effects in healthy subjects or in patients with class II–IV CHF from CAD.
This study has several limitations. The images were recorded over 3.5 h; thus, it is possible that the catecholamine state changed and affected our estimates of sympathetic function. Our prior studies in healthy subjects demonstrated no significant circadian variability in supine resting plasma NE or epinephrine (39). The lack of change in any hemodynamic parameter beyond a slight decrease in resting heart rate during the imaging period suggests that no major alteration occurred in sympathetic function.
Four subjects were taking amiodarone, which theoretically could interfere with 11C-mHED kinetics. However, it was recently shown that amiodarone actually improves NE uptake and retention in rats with chronic heart failure as compared with that of healthy rats (40). Thus, it seems likely that amiodarone did not exaggerate mismatch and potentially could have produced higher 11C-mHED uptake and a reduced mismatch than might have been otherwise observed.
Eight of our subjects were taking β-blockers (metoprolol), which could have interfered with our estimate of BAR density; however, none had received any for at least 24 h. The short plasma half-life (3.5 h) and binding of metoprolol should have cleared the metoprolol from the BAR in < 24 h. Analysis of the subjects on and off β-blockers suggests that there was no effect but we cannot prove this unequivocally. Anecdotally, we studied 1 patient who had received a β-blocker within 3 h of the study and there was no myocardial CGP uptake.
We did not correct the PET images for partial volume in regions in which the LV walls may have been thinned secondary to infarction. We did not have echo data for wall thickness in all subjects and, thus, did not attempt to use wall thickness for partial-volume correction. However, partial volume should not differ greatly between mHED and CGP as both were labeled with 11C. We excluded from analysis infracted regions in which the partial-volume effect was likely to be greatest.
Finally, there were few adverse events and the endpoint of transplantation for progressive CHF is subjective because transplantation depends on availability of an appropriate donor heart and no contraindications. For the patient in whom transplantation was the endpoint, CHF had slowly worsened for several months before the PET study despite optimal medical therapy. His physicians opined that he would have died within a short time or required a mechanical assist device had not a heart become available. The other endpoint of ventricular arrhythmia leading to ICD discharge in 1 and death in the other are objective events, as was the 1 death from progressive CHF. Similar criteria were used by Pietila et al. (38).
CONCLUSION
This PET study demonstrates that global and regional presynaptic function is decreased in patients with ischemic CHF as compared with age-matched healthy subjects, whereas postsynaptic BAR density is decreased, although not significantly. This results in a mismatch between pre- and postsynaptic function that could produce local myocardial conditions that are arrhythmogenic or a marker of worsening CHF. Our preliminary study suggests that patients with the greatest mismatch had more adverse events. We also demonstrated that such PET can be done within a short time period that minimizes the potential for changes in sympathetic function, is clinically acceptable, and does not cause clinically significant hemodynamic changes in patients with moderately severe CHF. These observations are intriguing but require evaluation and confirmation in a much larger patient population before being considered in the clinical management of patients with CHF.
Acknowledgments
We thank Marilou Gronka, Katherine Seymour, and Janet May for data collection and image analysis; Barbara Lewellen and the PET suite personnel for image acquisition; Steve Shoner and Minna Zheng for radiochemistry; and Werner Stuetzle, PhD, for statistical support. This research was supported by NIH grants RO1 HL50239 and AG15462. None of the authors has any known conflicts of interest or financial relationships.
Footnotes
-
COPYRIGHT © 2008 by the Society of Nuclear Medicine, Inc.
References
- Received for publication June 19, 2007.
- Accepted for publication October 25, 2007.