Abstract
In vitro assessment of hormone receptor status using a ligand-binding assay or immunohistochemistry in breast cancer patients predicts endocrine responsiveness with an accuracy of only 60%–70%. Assessment of an end product of estrogen receptor stimulation, such as the progesterone receptor, is assumed to provide a measure of functional receptor content and has proven to increase predictive accuracy. In analogy with the estrogen-dependent regulation of somatostatin receptor (SSTR) expression in endocrine-responsive human breast cancer cell lines, efficient antiestrogen treatment in patients may result in a downregulation of SSTR at the cell surface in breast tumors. In vivo imaging of this molecular event by means of sequential 99mTc-depreotide scintigraphy could enable selection of breast cancer patients susceptible to endocrine therapy. Methods: Twenty patients with a diagnosis of advanced breast cancer in whom first- or second-line hormonal therapy was going to be initiated were included. Patients underwent sequential 99mTc-depreotide scintigraphy before and 3 wk after initiating hormonal treatment. Follow-up data were retrieved from routine clinical evaluation by means of physical examination, imaging (e.g., bone scan, CT, MRI) and blood analysis. Lesion-to-background ratios (L/BGs) were calculated on planar and SPECT images and a change of >25% between the baseline and follow-up scan was considered significant. Results: At 6 mo after initiation of treatment, 8 patients had stable disease and were considered to be responding to hormonal treatment, whereas 10 patients had progressive disease and were considered to be nonresponders. The positive and negative predictive values of baseline 99mTc-depreotide scintigraphy for endocrine responsiveness were 73% (8/11) and 100% (7/7), respectively. Sequential scans were always both positive or both negative. The relative change in 99mTc-depreotide uptake between sequential scans significantly differed in responders compared with nonresponders (P= 0.017)—uptake decreased in the first group and increased in the latter. As such, baseline 99mTc-depreotide scintigraphy combined with the changes in tracer uptake between the baseline and follow-up scan predicted endocrine responsiveness with an accuracy of 100%. Conclusion: Sequential 99mTc-depreotide scintigraphy could allow for separation of responders and nonresponders immediately or as early as 3 wk after initiation of treatment.
Hormone-dependent breast tumors are characterized primarily by a functional and intact estrogen receptor (ER) system. Antiestrogen agents selectively target the ER pathway to abolish its effect on cell growth. Because only about one third of breast cancer patients initially respond to endocrine therapy, there is a need for patient selection. So far, the only predictive factor approved for clinical routine use is the in vitro assessment of hormone receptor status on tissue samples by a ligand-binding assay (LBA) or immunohistochemistry (IHC). Still, only 50%–60% of patients with ER-positive tumors show response to hormonal therapy, whereas lack of detectable ERs is usually associated with 5%–10% response (1).
Possible causes of discordance between hormone receptor status and endocrine responsiveness include limits inherent to the available techniques (IHC, LBA) for hormone receptor assessment, such as sampling error and variations in specificity (animal dependent) seen with the preparation of antibodies. Scintigraphy could circumvent these inaccuracies and, moreover, offers the advantage of noninvasive and repetitive whole-body (WB) evaluation.
Assessment of hormone receptor status is usually based on tissue samples from the primary tumor, whereas this information is being used years or decades later in the metastatic situation, when the biology of the tumor might have changed (2). Although the whole tumor appears to be histopathologically homogeneous, ERs are often displayed only in certain tumor regions (3). Metastatic lesions originating from an ER-negative clone may be ER negative—nevertheless, the primary tumor is ER positive. Scintigraphy has the ability to address heterogeneity of metastatic receptor expression without tissue manipulation and provides an in vivo image of all disease sites at the moment of treatment need.
Another explanation for the lack of response to endocrine treatment in ER-positive disease is nonfunctioning of the ER. These receptors are recognized and measured by IHC, but in such tumors steroid hormone occupancy of the receptor is not the drive for cellular proliferation and hence antiestrogen therapy is inefficient. Assessment of the end product of ER stimulation, such as the progesterone receptor (PgR receptor), could bypass this problem and has proven to increase predictive accuracy (4,5). Experimental data suggest that the somatostatin receptor (SSTR) is an estrogen response element (6,7). In human endocrine-responsive breast cancer cells, estradiol (E2) stimulation of functional ER results in upregulation of SSTR subtype 2 (SSTR2) at the cell surface. Alternatively, blocking the ER by means of an antiestrogen leads to a decrease of SSTR expression. About 50%–75% of breast tumors are SSTR positive and express predominantly SSTR2 (8–11). SSTR expression is associated with ER positivity and hormone responsiveness and, hence, with a better clinical prognosis (3,12). Hypothetically, in analogy with the in vitro findings, efficient antiestrogen treatment of patients with metastasized breast cancer may result in downregulation of SSTR at the cell-surface level, which could be visualized in vivo using sequential SSTR scintigraphy.
In vivo imaging of SSTR-positive tumors is routinely performed using [111In-DTPA-d-Phe1]octreotide scintigraphy (Octreoscan [111In-pentetreotide]; Mallinkrodt) (13). However, technetium labeling offers clinical advantages when compared with indium labeling, including lower cost, better availability, and faster tumoral visualization enabling a 1-d protocol. Depreotide (NeoSpect/NeoTect [99mTc-P829]; Amersham Health/GE Healthcare) is a 99mTc-labeled somatostatin analog. It is a cyclic decapeptide with high affinity for SSTR2, -3, and -5 (14–16).
This study was undertaken to evaluate the potential of sequential 99mTc-depreotide scintigraphy to select patients likely to respond to endocrine therapy.
MATERIALS AND METHODS
Patients
This study was approved by the Medical Ethics Committee of Ghent University Hospital and performed according to good clinical practice. All subjects gave their written informed consent before participation in the study. Twenty patients with advanced breast cancer in whom first- or second-line hormonal therapy was going to be initiated were included. One patient received chemotherapy immediately after starting hormonal treatment and endocrine therapy was eventually not initiated in 1 patient; both patients were excluded. As such, 18 patients were eligible for the study and patient characteristics are summarized in Table 1. All patients were female and had a mean age of 63 y (range, 46–76 y). One patient had a first diagnosis of locally advanced breast cancer and 17 patients had progression of previously treated breast cancer, all detected by plural diagnostic procedures (e.g., bone scan, CT, MRI, ultrasound, x-rays). The time to initial diagnosis in the latter patients was 72 ± 68 mo. Patients underwent sequential 99mTc-depreotide scintigraphy before (baseline) and 3 wk after (follow-up) initiating hormonal treatment. This timing was chosen because steady-state intratumoural concentrations of tamoxifen are usually achieved after 2 wk of daily tamoxifen administration (17). Plasma concentrations of the aromatase inhibitors anastrozole and letrozole approach steady-state levels at about 7 d and 2–6 wk, respectively, of once-daily dosing (18, 19). Two patients had only a baseline scan. Follow-up data were retrieved from routine clinical evaluation by means of physical examination, imaging (e.g., bone scan, CT, MRI, ultrasound, x-rays) and blood analysis. Response evaluation was assessed according to RECIST (Response Evaluation Criteria of Solid Tumors) guidelines (20). Progressive disease was defined as an increase in the number of nonmeasurable lesions (e.g., bone lesions) or an increase in the number or size (>20%) of measurable lesions (e.g., liver lesions). Disease status was considered stable in the absence of both progressive disease and a serum rise in tumor marker (21). Because durable stable disease appears to be a clinically useful criterion of therapeutic remission, patients with stable disease for 6 mo or more were considered to be responding to hormonal treatment (22,23). Patients with disease progression within 6 mo were considered to be nonresponders.
Summary of Patient Characteristics, Imaging Data, and Follow-up
Radiopharmaceutical Synthesis
NeoSpect kits were kindly provided by Amersham Health (now part of GE Healthcare) and prepared according to the manufacturer's guidelines.
Imaging Studies
Planar WB and SPECT imaging was performed 4 h after intravenous injection of 555–740 MBq (15–20 mCi) 99mTc-depreotide. Total injected activity was calculated on the basis of the syringe activity before and after injection measured in a NaI γ-counter. Images were acquired using a double-head or a triple-head γ-camera (Axis and Irix, respectively; Marconi/Picker), equipped with low-energy, high-resolution, parallel-hole collimators. The energy peak was centered at 140 keV with a 15% window.
For WB imaging, subjects were positioned supine with their arms alongside their body and a point source of 3.7 MBq in a 5-mL syringe was placed in a phantom between their feet. Acquisition was performed simultaneously in anterior and posterior positions with a scan speed of 15 cm/min. Matrix size was 256 × 1,024 pixels.
For SPECT, patients were positioned supine and at times of imaging of the thoracic region with the arms raised alongside the head. Images were acquired over 15 min by 40 views of 20 s per detector with the triple-head γ-camera and over 23 min by 60 views of 20 s per detector with the dual-head γ-camera (120 steps; 3° per step; matrix size, 128 × 128). Transversal, coronal, and sagittal slices were reconstructed iteratively using the OSEM (ordered-subset expectation maximization) algorithm with 2 iterations and 6 subsets and postfiltered using a Butterworth filter (cutoff frequency, 1.2 cycle/cm; order, 5).
Data Analysis
For semiquantification of radioactivity uptake after injection of 99mTc-depreotide, regions of interest (ROIs) were drawn over lesions and background on planar scans and SPECT images if available. As background, a region over the lower part of the upper leg was chosen on planar scans and a region adjacent to the lesion was chosen on SPECT images. The shapes and sizes—that is, number of pixels—were kept constant for the baseline and follow-up scan. For each ROI, the geometric mean, corrected for physical decay, of total anterior and posterior counts was calculated. The lesion-to-background ratio (L/BG) was calculated for each lesion on planar images and, for SPECT images, the activity ratio was calculated in several consecutive slices for each lesion and averaged. In addition, uptake in tumor lesions was expressed as the percentage of injected dose ( %ID) calculated on planar images. Because changes in %ID and L/BG were equivalent in the same lesion on sequential scans and assessment of L/BG is simpler and less subject to errors compared with %ID, we further expressed tracer uptake solely as L/BG. Change in uptake between the baseline and follow-up scan as the percentage of initial uptake on the baseline scan was recorded. A change of >25% between the baseline and follow-up scan was considered significant. This cutoff of 25% was chosen on the basis of the uptake measured in a series of organs and soft tissue over sequential scans that never varied more than 25% within the same patient. This is in agreement with biologic variations observed in tracer kinetics and the reported reproducibility of nuclear imaging techniques in general (24,25).
Statistical Analysis
Absolute 99mTc-depreotide uptake on the baseline scan and relative changes in tracer uptake between baseline and follow-up scan were compared in responders and nonresponders with use of the Mann–Whitney U test.
RESULTS
Tables 1 and 2 summarize the results of the study. The mean follow-up interval was 20 mo (range, 8–35 mo). Four patients died during this follow-up period. At 6 mo after initiation of treatment, 8 patients had stable disease and were considered to be responding to hormonal treatment, whereas 10 patients had progressive disease and were considered to be nonresponders. Of 9 patients receiving first-line hormonal treatment, 5 (56%) responded, of 8 patients with acquired endocrine resistance 3 (38%) responded to second line antiestrogen therapy. The 1 patient that initiated third line endocrine treatment did not respond.
Data on Tracer Uptake and Change in Tracer Uptake on Sequential 99mTc-Depreotide Scintigraphy in Responders and Nonresponders
99mTc-Depreotide scintigraphy assessed before initiation of antiestrogen treatment was positive in all (or major part of) lesions in 11 (61%) patients (Fig. 1). Contrast of lesions is less compared with 99mTc-methylene diphosphonate (MDP) bone scan because of relatively high background activity in the bone marrow. Visualization of lesions situated in the low thoracic and high lumbar spine is hampered on planar images because of high physiologic uptake in liver, spleen, and kidneys. In some patients, not all lesions could be evaluated because cross-sectional SPECT images were not available for all lesions. In one of the latter patients (patient 17), on planar images, tracer uptake was initially noted only in the primary tumor because relatively uniform tracer uptake in the fully invaded spine mimicked the high physiologic uptake of 99mTc-depreotide in normal bone marrow. Upon fusion of MRI with sagittal SPECT images, bulky lesions coincided with regions of enhanced tracer uptake on SPECT (Fig. 2). All responders had positive baseline scans and 7 of 10 nonresponders had negative baseline scans. Thus, all patients with a negative scan did not respond to therapy (Fig. 3). The positive and negative predictive values of baseline 99mTc-depreotide scintigraphy for endocrine responsiveness were 73% (8/11) and 100% (7/7), respectively. Tracer uptake (expressed as L/BG) on the baseline scan was higher for responders compared with that of nonresponders, with a median [25th–75th percentile] of 4.43 [3.74–5.16], respectively, and 2.54 [2.37–4.36]; however, this difference was not significant (P= 0.102) (Fig. 4A).
A 57-y-old woman (patient 15) presented with bone metastasis and achieved sustained disease stabilization on endocrine treatment. (A) 99mTc-Methylene diphosphonate bone scan shows multiple metastatic lesions, among others, of the skull (arrowhead), shoulder (thin arrow), and pelvic (thick arrow) region. (B) 99mTc-Depreotide scintigraphy, assessed before initiation of tamoxifen, shows uptake in the respective pathologic lesions.
A 59-y-old woman (patient 17) with progression of bone metastasis on tamoxifen underwent 99mTc-depreotide scintigraphy before she switched to second-line endocrine treatment. (A) Posterior view of planar 99mTc-depreotide scintigraphy shows relatively uniform tracer uptake in fully invaded spine, mimicking the high physiologic uptake of 99mTc-depreotide in normal bone marrow. (B) Sagittal MR image depicting extensive tumoral invasion of full spine with total destruction of corpus of thoracic vertebra T11 (arrowhead) and bulky lesion at T5−T6 (arrow). (C and D) Upon fusion with sagittal SPECT images, these lesions coincide with regions of enhanced tracer uptake.
(A) 74-y-old woman (patient 1), under tamoxifen treatment, presented with positive bone scan showing multiple lesions in vertebral spine T3 and ribs, T9 (small arrows), right sacroiliac region (thick arrow), and left femur (circle). (B) 99mTc-Depreotide scintigraphy, assessed before switch to second-line hormonal therapy, was negative. She was a nonresponder and the number of bone lesions increased.
(A) Scatter plot of 99mTc-depreotide uptake (expressed as L/BG) on baseline scan, averaged over all lesions, in nonresponders and responders. (B) Scatter plot of relative change in tracer uptake (expressed as percentage of uptake on baseline scan) on sequential scans, averaged over all lesions, in nonresponders and responders. (C) Scatter plot of relative change in tracer uptake (expressed as percentage of tracer uptake on baseline scan) on sequential scans, in nonresponders and responders. Change in uptake per lesion was classified into 3 groups (increase, stable, decrease) using a cutoff of 25% and averaged per group for each patient.
Sequential scans, acquired before and 3 wk after initiation of antiestrogen treatment, were always both positive or both negative. The relative change in 99mTc-depreotide uptake between sequential scans significantly differed in responders compared with that of nonresponders (P= 0.017)—uptake decreased in the first group and increased in the latter. The median [25th–75th percentile] change in L/BG on the follow-up scan compared with the baseline scan was −19% [−33% to 1%] for responders and 34% [29%–116%] for nonresponders (Fig. 4B).
A cutoff of 25% was considered to define a significant increase or decrease in tracer uptake (Fig. 4C). Uptake that changed with <25% compared with the uptake on the baseline scan was considered as stable. In the group of responders, lesion uptake was stable in 3 patients, L/BG decreased in 2 patients, and in 2 patients uptake decreased in some lesions and was stable in others (Fig. 5). In 1 responding patient, only the baseline scan was acquired and, as such, change in uptake could not be assessed. In contrast, of the nonresponders with a positive scan, L/BG increased in 1 patient and in 2 patients uptake increased in some lesions and was stable in others. As such, baseline 99mTc-depreotide scintigraphy combined with the changes in tracer uptake between the baseline and follow-up scan predicted endocrine responsiveness with an accuracy of 100%.
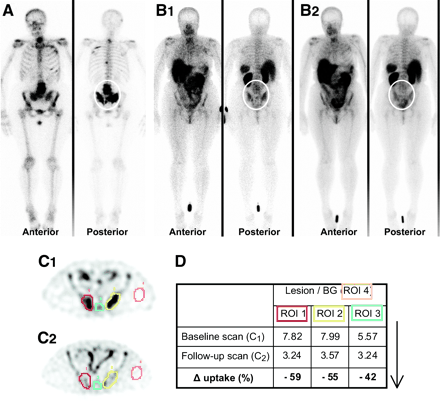
(A) A 61-y-old woman (patient 11) presented with multiple lesions on bone scan and had stable disease for 31 mo on tamoxifen treatment. (B) Sequential 99mTc-depreotide scintigraphy visualizes similar extensive bone metastasis as on bone scan, among others, in lumbar (L2–L5) and sacroiliac (B1, baseline scan; B2, follow-up scan) region. (C) ROIs were drawn over lesions (ROIs 1–3) and background (BG, ROI 4) on transverse SPECT images and measured counts were averaged over several slices of total tumor volume (C1, baseline scan; C2, follow-up scan). (D) L/BG ratios and change in uptake (Δ uptake, expressed as percentage of uptake first scan) were calculated. 99mTc-Depreotide uptake significantly decreased with values of −42% to −59%.
DISCUSSION
Endocrine therapy is one of primary treatment options for most patients with metastatic breast cancer. Upon patient selection based on hormone receptor status, 50%–75% of patients with ER- or PgR-expressing tumors initially respond. Nearly all responding patients eventually have disease progression, but approximately half of patients with acquired resistance obtain a clinical benefit from other endocrine therapies. Although response rates progressively decline, they remain in the 20%–40% range (26). Our limited patient data are concordant with these reported figures for sequential endocrine responsiveness.
In our study, all patients with hormone-sensitive tumors had positive scans. This could be expected since E2 stimulation of functional ER enhances SSTR2 gene transcription and, hence, SSTR2 expression at the cell surface, which is visualized by 99mTc-depreotide scintigraphy. In this group of responders, tracer uptake on the follow-up scan tended to be lower compared with that on the baseline scan and, considering a cutoff of 25%, uptake significantly decreased or remained stable. According to our hypothesis, this decrease could be interpreted as a downregulation of SSTR2 at the cell-surface level caused by an efficient block of the functional ER that inhibits further transcription of the SSTR2. Unfortunately, because of availability reasons, binding of radioligand to breast tumor cells per se could not be confirmed using autoradiography. Therefore, binding of 99mTc-depreotide to other kinds of cells expressing SSTR2, -3, or -5, such as activated lymphocytes (subtype 2), cannot be excluded (27). The presence of tumor-infiltrating lymphocytes has been associated with therapy response and good prognosis; however, controversy exists (28,29). Equivalent uptake on both sequential scans could be interpreted as absence of active stimulation of ER and, subsequently, absence of SSTR2 transcription. Possibly, intratumoral levels of antiestrogen could have only just achieved or not yet achieved steady state and balance between receptor synthesis and endocytosis/degradation has not yet turned in favor of degradation. However, 1 patient underwent anastrozole administration (patient 13), which achieves steady state within 7 d (18); however, this relates to serum levels and not concentrations in the respective tissues of action.
Three patients of the group of responders had acquired resistance to tamoxifen and had positive 99mTc-depreotide scans before they switched to a second-line aromatase inhibitor. This is in agreement with a considerable amount of data indicating that ER in acquired resistant breast cancer commonly remains functional and, moreover, pivotal to the growth regulation and gene expression profile of breast tumors on their relapse, despite the presence of tamoxifen (30). Clinical experience has shown that hormonal resistance is often reversible, suggesting a cellular adaptation, rather than genetic alterations in many breast cancer patients (31). Changes in local metabolism and, in particular, more reduced concentrations of tamoxifen and its metabolites are reported in tamoxifen-resistant tumors (32). Accordingly, efflux of tamoxifen out of the tumor cell in the presence of a functional ER results in a tamoxifen-resistant tumor, expressing estrogen-regulated genes (e.g., SSTR2), sensitive to aromatase inhibitors. Besides prediction of the likelihood of a response to second-line hormonal treatment such as aromatase inhibitors requiring a functional ER, this could allow prediction of early resistance to tamoxifen treatment.
All negative 99mTc-depreotide scans belonged to patients with tumors resistant to hormonal therapy. This was in the line of expectations since we anticipated a nonfunctional ER, not capable of stimulating transcription and expression of SSTR2. Loss of ER is generally not a feature of acquired endocrine resistance either in vitro or in vivo; hence, repeated hormone receptor assessment using routine techniques (LBA, IHC) for determination of endocrine responsiveness would be inadequate (33).
Three patients (30%) of the group of nonresponders had positive 99mTc-depreotide scintigraphies. This suggests a ligand-independent stimulation of ER and estrogen-independent regulation of SSTR2 expression. Constitutively active ER variants might contribute to a tamoxifen-resistant breast tumor with similar characteristics; however, mutations are extremely rare in vivo and, thus, refer only to a minority of cases (34). Even in tumors that are estrogen dependent, it is likely that an appropriate growth factor environment is necessary for efficient mitogenesis, with steroid hormone and growth factor signaling pathways “cross talking” to reinforce each other's signaling (35,36). Perturbance of the balance of steroid hormone and growth factor interaction can result in endocrine resistance, among others, due to tamoxifen acquiring more agonist-like activity. The resulting ER-mediated stimulation of SSTR2 expression could cause an increase in tracer uptake on the follow-up scan. However, in these 3 patients, endocrine treatment with an aromatase inhibitor was initiated.
Presumably, regulation of SSTR expression in breast tumor cells is not exclusively estrogen dependent and is influenced by a series of other mediators, as is the case in normal tissue (e.g., pancreas, hypothalamus) (37). Growth factors (transforming growth factor β, insulin-like growth factor 1) and cytokines (interleukin 6, tumor necrosis factor α) secreted by tumor cells or adjacent host cells may modify SSTR expression in an autocrine or paracrine manner (38,39).
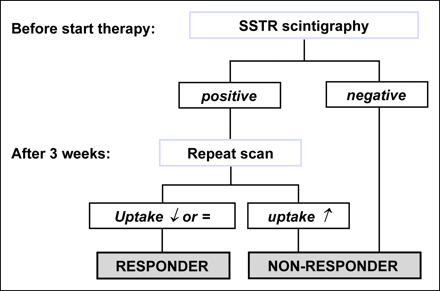
The small number of patients included limit the study results. Nevertheless, we found a consistent relationship between 99mTc-depreotide uptake and response to endocrine treatment in metastasized breast cancer patients. The underlying mechanism is suggested to be an ER-mediated regulation of SSTR2 expression but an additional influence by a series of cytokines or growth factors present in the tumoral environment is to be expected. If a larger-scale study confirms these findings, this could entail a powerful tool to accurately evaluate endocrine responsiveness. A protocol with SSTR scintigraphy before initiation of endocrine treatment and repetition of the scan if positive could be proposed (Fig. 6).
Proposed protocol for selection of advanced breast cancer patients for endocrine treatment using sequential 99mTc-depreotide scintigraphy.
Realistic goals for treatment of metastatic breast cancer are palliation of symptoms and prolongation of survival with maximization of the quality of life. In general, significant palliation is more likely for a patient who experiences a response to endocrine therapy compared with a similar response induced by chemotherapy, due to a lower toxicity profile. A delay in effective therapy may lead to a decline in performance status and organ function and, as such, reduce the likelihood of a subsequent response. With the use of clinical follow-up and conventional morphologic imaging modalities using volumetric changes, easily 3–6 mo are needed for response evaluation.
CONCLUSION
The proposed protocol (Fig. 6) would allow for separation of responders and nonresponders immediately (negative baseline scan) or as early as 3 wk after initiation of treatment (positive baseline scan).
Acknowledgments
This project was supported by a Fund for Scientific Research grant of the Ghent University and the Flemish Government (G.0029.02.). Christophe Van de Wiele is holder of a research mandate of the Fund for Scientific Research–Flanders (Belgium) (FWO–Vlaanderen).
Footnotes
-
↵† Deceased.
References
- Received for publication August 8, 2005.
- Accepted for publication October 7, 2005.