Abstract
We investigated the reproducibility of an automatic quantitative algorithm for measuring regional myocardial wall motion and systolic thickening. Methods: 99mTc-sestamibi gated myocardial SPECT with dipyridamole stress was performed twice consecutively on 31 patients with known or suspected coronary artery disease, with the patients in the same position for each scan. With AutoQUANT software, segmental wall motion and systolic thickening were quantified automatically and expressed in millimeters and percentage increase, respectively, for 20 segments. Afterward, the correlation and agreement between repeated measurements were investigated, and the influences of wall location, perfusion grade, and partitioning of the myocardium on reproducibility were evaluated by ANOVA and t testing. Results: High correlations (r = 0.95 for wall motion and 0.88 for systolic thickening) and good agreements (weighted κ = 0.81 and 0.71, respectively) were obtained from repeated measurements on consecutive gated SPECT. Changes in wall location and perfusion grade did not cause significant differences between repeated measurements (P > 0.05 in ANOVA and t testing), but a change in partitioning did. On Bland–Altman analysis, 2 SDs for repeated wall motion and for systolic thickening were 2.0 mm and 20%, respectively. Conclusion: The automatic quantitative algorithm for myocardial SPECT provided by AutoQUANT software has good reproducibility under diverse conditions. A change of motion > 2.0 mm or a change of systolic thickening > 20% can be regarded as significant during a follow-up study using this software.
Regional myocardial function and perfusion are important in coronary artery disease because they indicate myocardial viability and predict prognosis after revascularization at the regional level (1–3). Myocardial wall motion and systolic thickening are frequently used parameters for regional myocardial function. They can be assessed by several methods, such as gated SPECT, echocardiography, or cine MRI (4–7). With gated SPECT, myocardial function and perfusion can be assessed simultaneously (8), and the findings have a reputation of reliability compared with those of other methods (9–11).
Algorithms extracting myocardial boundaries from end-systolic and end-diastolic images have improved the assessment of regional myocardial function on gated SPECT (12,13). On the basis of those algorithms, Germano et al. (14) proposed a new algorithm for the automatic quantitation of myocardial wall motion and systolic thickening on gated SPECT and reported that the algorithm agreed well with visual assessment. The algorithm provides quantitative values without manual intervention, whereas most visual assessment is semiquantitative. This automatic algorithm also has the advantage of being independent of the observer’s subjectivity. When an automatic algorithm provides consistent measurements from a gated SPECT image, the algorithm can be considered to provide almost perfect intraobserver reproducibility.
However, the reproducibility of measurements acquired under the same conditions is also important, and normal variations between conditions should be investigated (15). This point is particularly critical when gated SPECT is used for a follow-up study or for evaluating the effect of some treatment. The same clinical condition can be assumed if data are acquired from a patient consecutively in a short time without any intervening treatment or change of position. In this study, gated SPECT images were acquired in that manner, and myocardial motion and systolic thickening were quantified using software incorporating the automatic algorithm.
Reproducibility should be guaranteed under diverse clinical conditions. Several factors may interfere with the interpretation of SPECT images. Attenuation affects image quality and the interpretation of myocardial SPECT (16,17). Each wall location has different attenuation characteristics, and these may affect images. Decreased perfusion makes extraction of myocardial boundaries difficult (18). Partitioning is a process in the automatic algorithm itself and is an important factor when assessing regional parameters.
In this study, the reproducibility of the automatic algorithm for measuring regional myocardial motion and systolic thickening was investigated. The influences of some likely affecting factors, such as wall location, perfusion, and partitioning of the myocardium, on reproducibility were also evaluated.
MATERIALS AND METHODS
Patient Population
Thirty-one patients (23 men, 8 women; age range, 32–73 y; mean age ± SD, 59.2 ± 9.7 y) with known or suspected coronary artery disease were enrolled in this study. Four patients had a history of old myocardial infarction (20 ± 14 mo before study), and another 4 patients had a history of recent myocardial infarction (12 ± 7 d before study). Coronary angiography was performed on 21 patients, and all had coronary artery stenosis occluding more than 50% of the intravascular diameter (4 of a single vessel, 6 of 2 vessels, and 11 of 3 vessels). The stenosis was in the left anterior descending artery in 19 patients, in the left circumflex artery in 15 patients, and in the right coronary artery in 15 patients. Five patients had a history of coronary artery bypass grafting, and another 5 patients had a history of percutaneous transluminal coronary angioplasty before the study. Left ventricular ejection fraction was 51% ± 14%, and end-systolic volume was 65 ± 40 mL.
Image Acquisition
During dipyridamole stress, 925 MBq 99mTc-sestamibi were injected. After 1 h, gated 99mTc-sestamibi SPECT was performed and another acquisition was obtained consecutively without any intervening treatment or change of patient positioning (Fig. 1). Sixteen frames of data according to the averaged cardiac cycle were acquired with a 40% window centered over the 140-keV photopeak, using a dual-head 90° camera (Vertex; ADAC Laboratories, Milpitas, CA). A ramp filter and a Butterworth low-pass filter of order 5 and cutoff frequency 0.33 cycle/pixel were used for backprojection. No attenuation or scatter correction was done, and tomographic transaxial images and gated surface images were reconstructed from the backprojection.
Procedure for dipyridamole stress 99mTc-sestamibi (MIBI) gated SPECT. Scanning was performed twice consecutively in short time without change of position.
Automatic Quantitation
The myocardium was divided into 20 segments (Fig. 2). In each of the 20 segments, the average quantitative values of segmental wall motion and systolic thickening were determined using AutoQUANT software (ADAC Laboratories). Wall motion was calculated and expressed in millimeters, and systolic thickening was expressed as percentage of end-diastolic wall thickness. The entire process was performed automatically, without manual intervention.
Myocardium was partitioned into 20 segments. Apex was divided into 2 segments, and apical, mid, and basal regions were divided into 6 segments each. For assessment of influence of wall location, segments were classified into 5 wall locations. Each pattern represents different wall location.
The influence of partitioning on reproducibility was evaluated by repartitioning the myocardium into 5 regions (apex, anterior, lateral, inferior, and septal walls), and average regional motion and systolic thickening were also quantified in each of these regions. These measurements were also acquired automatically using AutoQUANT software.
Perfusion Grading
For each of the 20 segments, perfusion was evaluated automatically using AutoQUANT software, which awarded a quantitative value and scored the segment according to a 5-grade system (0 through 4). This algorithm has been reported to have good reproducibility and clinical relevance (19,20).
Statistical Analysis
Reproducibility was checked through calculation of correlation coefficients between repeated measurements. Because the correlation coefficient cannot properly represent agreement, weighted κ values were also calculated. For this calculation, the quantified values were scored using a 5-grade system for wall motion (0 = ≥6 mm, 1 = 4–5.9 mm, 2 = 2–3.9 mm, 3 = 0–1.9 mm, and 4 = <0 mm [Table 1]) and using a 4-grade system for systolic thickening (0 = ≥40%, 1 = 20%–40%, 2 = 10%–20%, and 3 = <10%). The range of differences between repeated measurements was shown on a Bland–Altman plot, and 2 SDs of the differences were calculated.
Agreement of Segmental Motion Grades from Automatic Quantified Values on Repeated Gated SPECT
ANOVA was performed to evaluate the influences of wall location and perfusion status. Twenty segments were classified into 5 wall locations (Fig. 2), and ANOVA was performed on the absolute differences between repeated measurements, setting wall location as an effect factor. ANOVA was also performed with perfusion grade set as an effect factor, and t testing was performed to evaluate the influence of myocardial partitioning. In every analysis, P < 0.05 was considered significant, and κ values were graded as follows: 0–0.20 = slight, 0.21–0.40 = moderate, 0.41–0.60 fair, and 0.61–1.00 = good to perfect (21).
RESULTS
General Reproducibility of Quantified Values
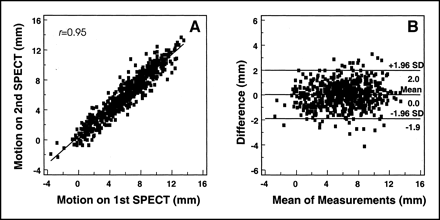
The mean value of myocardial wall motion was 5.9 ± 3.0 mm (range, −3.6 to 13.7 mm), and the correlation coefficient between repeated measurements was 0.95 (Fig. 3A). The Bland–Altman plot showed no deviation in dispersion of differences according to the means of their motion values, and 2 SDs of the differences covered a range of ±2.0 mm (Fig. 3B).
Correlation of automatic quantitation of motion on repeated gated SPECT (A) and Bland–Altman plotting of motion (B).
Mean systolic thickening was 34% ± 20% (range, −5% to 103%), and the correlation coefficient between repeated measurements was 0.88 (Fig. 4A). No deviation of dispersion occurred, and 2 SDs covered a range of ±20% (Fig. 4B).
Correlation of automatic quantitation of systolic thickening on repeated gated SPECT (A) and Bland–Altman plotting of systolic thickening (B).
κ values also exhibited good reproducibility. The weighted κ values for agreement between repeated measurements were 0.81 (95% confidence interval, 0.78–0.84) for wall motion (Table 1) and 0.71 (95% confidence interval, 0.67–0.75) for systolic thickening (Table 2), which represented good to perfect agreements.
Agreement of Segmental Thickening Grades from Automatic Quantified Values on Repeated Gated SPECT
Influence of Wall Location
The correlation coefficients for each wall location are shown in Table 3. Although those for systolic thickening in the septal and inferior walls were relatively low, the overall correlation was good in every location. The absolute differences between repeated measurements according to wall location are shown in Figure 5. By ANOVA, setting wall location as an effect factor, no significant distinction was found among wall locations. To take into account the different characteristics of the attenuation pattern between men and women, we performed the same analysis for each sex group. However, neither group showed significant distinction by wall location (Table 4).
Means and confidence intervals (CI) of absolute differences between repeated measurements for wall motion (A) and for systolic thickening (B) according to wall locations. Ant = anterior wall; Inf = inferior wall; Lat = lateral wall; Sept = septal wall.
Correlation Coefficients Between Repeated Measurements of Segmental Motion and Thickening in Each Wall Location and Perfusion Grade
Probability Values on ANOVA, Setting Wall Location or Perfusion Grade as an Effect Factor
Influence of Perfusion
When segments were classified by perfusion grade, 384 segments were grade 0, 108 were grade 1, 69 were grade 2, 32 were grade 3, and 27 were grade 4. The measurements of motion and thickening in each group are tabulated in Table 5. The correlation coefficients in each perfusion grade are shown in Table 3. Both segmental motion and systolic thickening correlated well between repeated measurements. Absolute differences according to perfusion grade are illustrated in Figure 6. Setting perfusion grade as an effect factor, the ANOVA of absolute differences between repeated measurements showed no significant distinction among the perfusion groups for either wall motion or systolic thickening. This finding was retested for each sex group. In this analysis, no significant distinction was found (Table 4).
Means and confidence intervals (CI) of absolute differences between repeated measurements for wall motion (A) and for systolic thickening (B) according to perfusion grade. Higher grade represents more severe perfusion decrease.
Measurements of Motion and Thickening According to Perfusion Grade
Influence of Partitioning
In segmental data, the absolute differences between repeated measurements were 0.77 ± 0.62 mm for wall motion and 7.2% ± 7.2% for systolic thickening. After the myocardium was repartitioned into 5 wall regions, wall motion and systolic thickening were assessed in each region. The absolute differences were 0.52 ± 0.49 mm for wall motion and 4.5% ± 3.7% for systolic thickening. The absolute differences between the 2 groups were significant on t testing (P < 0.001). Partitioning the myocardium into larger regions showed smaller absolute differences between repeated measurements.
DISCUSSION
This study showed good reproducibility for the automatic quantitation of regional myocardial motion and systolic thickening. In consecutive studies using quantitative gated SPECT software (Cedars-Sinai Medical Center, Los Angeles, CA), the reproducibility of the visual assessment of myocardial motion and thickening was reported to have κ values of 0.76 and 0.87, respectively (22). The automatic algorithms investigated in this study showed similar results. Both motion and thickening showed high agreement for repeated measurements.
Visual assessment of motion or thickening was reported to be less reproducible for dysfunctional myocardium than for normal myocardium (23). However, the Bland–Altman plot in this study showed no distinction between groups with better functioning and groups with worse functioning, meaning that the functional status does not affect the reproducibility of this algorithm. This advantage is another benefit of the automatic algorithm.
Patterns of attenuation differ with wall location (24); moreover, myocardial motion and systolic thickening have been reported to show different normal variations according to wall location (25). Nevertheless, this study showed no significant distinction between segments in different wall locations. In addition, no distinction was found between different perfusion groups. Although the probability value was near 0.05 and Figure 6 displays some distinctive patterns between the various perfusion groups, these patterns seemed to be the result of some factor other than lower perfusion, because the group with worse perfusion showed the lower absolute differences. The fewer segments in the higher grade may have caused this result. A decreased radioactivity count caused by decreased perfusion or attenuation induces artifacts such as the partial-volume effect and makes the interpretation of the gated SPECT images difficult by both visual assessment and automatic algorithms (18,26). However, the perfusion and wall motion did not affect the reproducibility in this study.
Partitioning is an intra-algorithmic process composed of the reorientation of the acquired image and section. This process is one of the critical points in the automatic interpretation of myocardial SPECT (27,28). After the partitioning into larger regions, absolute differences between repeated measurements and their SDs decreased to a statistically significant degree. Therefore, improvement of the partitioning process will likely improve reproducibility.
Another concern of this study was normal variation in repeated measurements. To follow up a patient or evaluate the effect of treatment, we need to determine changes in myocardial function. Two SDs of differences between repeated measurements were ±2 mm for motion and ±20% for systolic thickening. One study found that 2 SDs of interobserver differences for systolic thickening were ±16%, when assessed using an automatic algorithm that extracts myocardial boundaries in echocardiography (29). Considering that those results were not obtained at the segmental level, they seem to be similar to the results of our study.
CONCLUSION
The automatic algorithm for quantitation of regional myocardial wall motion and systolic thickening showed good reproducibility and was not affected by such factors as myocardial functional status, attenuation, and perfusion. The absolute difference between repeated measurements was significantly decreased by a change in partitioning. Therefore, this algorithm can reliably be applied to diverse clinical conditions, but the partitioning process needs further discretion. Finally, when this algorithm is used in follow-up studies or in evaluating the effect of some management, changes in motion more than 2.0 mm and changes in systolic thickening more than 20% can be regarded as significant.
Footnotes
Received Nov. 20, 2000; revision accepted Mar. 16, 2001.
For correspondence or reprints contact: Dong Soo Lee, MD, PhD, Department of Nuclear Medicine, Seoul National University College of Medicine, 28 Yungundong Chongno-gu, Seoul, 110-744, Korea.