Abstract
18F-Fluoride PET/CT was performed on 44 oncologic patients to evaluate its diagnostic accuracy in assessing malignant osseous involvement and in differentiating malignant from benign bone lesions. Methods: 18F-Fluoride PET and 18F-fluoride PET/CT were interpreted separately. Lesions showing increased 18F-fluoride uptake were categorized as malignant, benign, or inconclusive. The final diagnosis of lesions was based on histopathology, correlation with contemporaneous diagnostic CT or MRI, or clinical follow-up of at least 6 mo (mean, 10 ± 3 mo). Results: Increased 18F-fluoride uptake was detected at 212 sites, including 111 malignant lesions, 89 benign lesions, and 12 lesions for which the final diagnosis could not be determined. In a lesion-based analysis, the sensitivity of PET alone in differentiating benign from malignant bone lesions was 72% when inconclusive lesions were considered false negative and 90% when inconclusive lesions were considered true positive. On PET/CT, 94 of 111 (85%) metastases presented as sites of increased uptake with corresponding lytic or sclerotic changes, and 16 of the 17 remaining metastases showed normal-appearing bone on CT, for an overall sensitivity of 99% for tumor detection. For only 1 metastasis was PET/CT misleading, suggesting the false diagnosis of a benign lesion. The specificity of PET/CT was significantly higher than that of PET alone (97% vs. 72%, P < 0.001). PET/CT identified benign abnormalities at the location exactly corresponding to the scintigraphic increased uptake for 85 of 89 (96%) benign lesions. In a patient-based analysis, the sensitivity of PET and PET/CT was 88% and 100%, respectively (P < 0.05) and the specificity was 56% and 88%, respectively (not statistically significant). Among the 12 patients referred for 18F-fluoride assessment because of bone pain despite negative findings on 99mTc-methylene diphosphonate bone scintigraphy, 18F-fluoride PET/CT suggested malignant bone involvement in all 4 patients with proven skeletal metastases, a potential benign cause in 4 of 7 patients who had no evidence of metastatic disease, and a soft-tissue tumor mass invading a sacral foramen in 1 patient. Conclusion: The results indicate that 18F-fluoride PET/CT is both sensitive and specific for the detection of lytic and sclerotic malignant lesions. It accurately differentiated malignant from benign bone lesions and possibly assisted in identifying a potential cause for bone pain in oncologic patients. For most lesions, the anatomic data provided by the low-dose CT of the PET/CT study obviates the performance of full-dose diagnostic CT for correlation purposes.
In 1962, 18F-fluoride was first introduced as a bone-imaging agent by Blau at al. (1). 18F-Fluoride PET imaging combines the superior pharmacokinetic properties of 18F-fluoride (compared with those of 99mTc-polyphosphonates) and the improved spatial resolution and lesion contrast of PET technology (compared with those of planar and even SPECT γ-camera imaging). 18F-Fluoride has higher bone uptake and faster blood clearance, resulting in a better target-to-background ratio (2–5). 18F-Fluoride PET has been shown to be more accurate than 99mTc-methylene diphosphonate (MDP) bone scintigraphy for the detection of both sclerotic and lytic lesions in various malignancies and was suggested as an alternative to bone scintigraphy, mainly in patients at high risk for metastatic bone disease but also in patients for whom the detection of metastatic bone disease and its extent is important in selection of treatment (6–8). In patients with lung cancer, for instance, Schirrmeister et al. (9) reported that 18F-fluoride PET detected bone metastases overlooked by SPECT bone scintigraphy. In that study, all metastatic lesions diagnosed using MRI were also detected by 18F-fluoride PET (9). Increased 18F-fluoride uptake is, however, not specific for tumoral bone involvement, and the high sensitivity of 18F-fluoride PET may also be associated with a higher detection rate of benign bone lesions. Even using a crude ratio of lesion to normal tissue, one cannot differentiate benign from malignant 18F-fluoride uptake (4). Correlation with morphologic imaging modalities, such as CT and MRI, is the most common approach for the characterization of scintigraphic bone lesions (6,7,10).
Integrated systems that allow PET and CT to be performed in the same setting and their images to be fused have recently been introduced clinically. Data suggest improved diagnostic accuracy for PET/CT in tumor detection using 18F-FDG as the tumor-imaging agent (11–14). The current study reports initial experience with 18F-fluoride PET/CT imaging in 44 patients with various malignant diseases and compares the diagnostic accuracy of 18F-fluoride PET and 18F-fluoride PET/CT in the assessment of malignant bone involvement.
MATERIALS AND METHODS
Patient Population
18F-Fluoride PET/CT was performed on 44 cancer patients (20 male and 24 female; mean age, 52 ± 17 y; range, 15–81 y) in our initial experience with this modality. 18F-Fluoride PET/CT was indicated to perform a metastatic survey (as an alternative to 99mTc-MDP bone scintigraphy) (n = 21), to investigate skeletal pain for which the results of bone scintigraphy were normal (bone scintigraphy was performed 1–6 wk before the 18F-fluoride study) (n = 12), and to investigate bone highly suggestive of tumoral involvement because of abnormal laboratory findings (e.g., elevated blood tumor markers) or unclear findings on other imaging modalities (n = 10). Consecutive patients who matched these indications and gave informed consent to participate were enrolled in the study. The patients had various oncologic diseases, including cancer of the breast (n = 10), prostate (n = 6), lung (n = 4), colon (n = 4), ovary (n = 1), nasopharynx (n = 1), and testes (n = 1); gastrointestinal stromal tumor (n = 2); lymphoma (n = 4); malignant melanoma (n = 2); multiple myeloma (n = 4); Ewing’s sarcoma (n = 3); soft-tissue sarcoma (n = 1); chondrosarcoma (n = 1); metastatic giant cell tumor (n = 1); and carcinoid (n = 1). Two patients had double primary tumors.
18F-Fluoride Preparation
18F-Fluoride was produced by the 18O(p,n)18F nuclear reaction on 2 mL of enriched 18O-water as a target and then transferred into a fluorination module by a flow of argon. After trapping, it was loaded on an anion exchange column (Chromafix; Macherey-Nagel), dried, eluted with 1 mL of K2CO3 solution (5 mg/mL), and transferred to the reactor. After the addition of 1 mL of sterile water, the solution was heated to 120°C (2 min) followed by evaporation under reduced pressure, lowering of temperature to 30°C, addition of 10 mL of saline and 4 mL of phosphate buffer (pH 7), and transfer into a product vial through a 0.22-μm sterile filter. Chemical and radiochemical purity was analyzed by anion exchange high-performance liquid chromatography on an IC-Pak anion high-resolution column (4.6 × 75 mm; Waters) eluted with sodium borate/gluconate solution and acetonitrile (20 mL of borate gluconate concentrate, 20 mL of n-butanol, 120 mL of acetonitrile, and 860 mL of deionized water) at a flow of 0.8 mL/min.
PET/CT Technique
No special preparations were needed before the 18F-fluoride PET/CT study. Scanning was performed 45 min after the intravenous administration of 296–444 MBq (8–12 mCi) of 18F-fluoride using a Discovery LS PET/CT system (GE Medical Systems). Low-dose CT was performed first with 140 kV, 80 mA, 0.8 s per CT rotation, a pitch of 6, and a table speed of 22.5 mm/s, without any specific breath-holding instructions. A PET emission scan was obtained immediately after acquisition of the CT scan, without changing the patient’s position. From 5 to 9 bed positions were used, with an acquisition time of 3 min for each, and the skeleton was imaged from the skull to the femurs. If lesions at the distal regions of the limbs were suspected before the PET/CT study, a second PET/CT study was performed to include these areas (3 patients). PET images were reconstructed using an ordered-subsets expectation maximization algorithm. CT data were used for attenuation correction. Studies were interpreted on an eNTEGRA workstation (GE Medical Systems).
18F-Fluoride PET/CT Interpretation
PET and PET/CT images were interpreted on 2 separate days, in a consensus reading by 2 individuals. The patient’s name was removed from each report, and the order of the reports was changed before the second interpretation. Readers were unaware of clinical data and the findings of other imaging modalities. Each site of abnormally increased 18F-fluoride uptake was recorded and rated on the PET and PET/CT images as benign, malignant, or inconclusive.
On the PET images, vertebral lesions were categorized as benign or malignant on the basis of the vertebral region involved. Lesions localized at the facet joints, endplates, beyond the vertebral body, or at the spinous process were considered benign, whereas those localized at the posterior part of the body or at the pedicles were considered malignant (15). Benignity of scintigraphic findings in the remainder of the skeleton was suggested if they were around the joints or had a pattern of uptake typical of fracture, such as a vertical line of increased uptake through multiple ribs, an H-shaped pelvic abnormality, or other findings often associated with benign causes (e.g., trochanteric bursitis, avulsion injury, or tendonitis). In the results analysis, only sites of increased uptake that could be either benign or malignant, thus raising a diagnostic dilemma, were included. Clearly benign lesions that are often seen on bone scintigraphy and do not pose such a dilemma (e.g., lesions in the acromioclavicular joints, lesions at the joints of the peripheral skeleton, and lesions caused by dental problems) were not included in the analysis.
On PET/CT images, lesions were categorized as benign if increased 18F-fluoride uptake correlated in location with a benign CT finding. Malignancy was suggested if increased uptake correlated in location with lytic, sclerotic, mixed lytic-sclerotic, or intramedullary changes (i.e., loss of bone marrow fat) on CT. In the initial analysis of results, only lesions associated with these types of CT changes were categorized as true positive for bone malignancy; if CT showed no abnormality at the location corresponding to the PET finding, the lesion was categorized as inconclusive. The rationale for these strict criteria was that, in practice, the lack of clear changes on CT may require further validation of the nature of the lesion, especially for a solitary lesion without clear evidence of bone malignancy elsewhere. In the repeated analysis, lesions associated with increased uptake and normal CT findings were considered malignant.
The final diagnosis of lesions was based on histopathology, imaging, and clinical follow-up of at least 6 mo (mean, 10 ± 3 mo; range, 6–15 mo). Imaging follow-up was performed with diagnostic full-dose CT, MRI, or 99mTc-MDP bone scintigraphy. 18F-FDG PET/CT was used as the standard of reference for the final diagnosis in 6 of the study patients. A lesion showing 18F-fluoride uptake was considered malignant if it also showed 18F-FDG uptake that either disappeared after chemotherapy or increased on a repeated study and if disease progression was clinically evident.
Statistical Analysis
The sensitivity and specificity of 18F-fluoride PET and 18F-fluoride PET/CT for the differentiation of malignant from benign bone lesions were assessed and compared in lesion-based and patient-based analyses using the McNemar test. P < 0.05 was considered statistically significant.
RESULTS
Lesion-Based Analysis
The results analysis included 212 sites of increased 18F-fluoride uptake. There were 111 malignant lesions and 89 benign lesions based on histopathology (n = 9), contemporaneous diagnostic CT or MRI (n = 64), or imaging and clinical follow-up (n = 125). The final diagnosis of the remaining 12 lesions could not be established; these represented inconclusive findings on PET/CT that were not further assessed because the patients had proven metastases elsewhere.
Of the 111 metastases, 38 were at the vertebral column, 17 at the thoracic cage (including the ribs, clavicle, sternum, and scapula), 29 at the pelvic bones,15 at the skull and facial bones, and 12 at the long bones of the extremities. For 94, an accurate diagnosis was made through PET/CT, which detected corresponding lytic changes on CT images for 43 lesions, sclerotic changes for 37, and mixed lytic and sclerotic changes for 14. In 12 of the malignant lesions, in addition to the bony changes a soft-tissue component was detected on CT as a soft-tissue mass either occupying the lytic space or adjacent to the bony lesion. Figures 1 and 2 illustrate the various PET/CT patterns of malignant bone involvement. In 16 of the 17 remaining malignant lesions, CT findings were normal, showing neither benign changes nor lytic or sclerotic changes. In only 1 of the 111 malignant lesions were the CT findings misleading, resulting in a benign diagnosis. In lytic metastases, the increased uptake was often at the margin of the lesion. Two such lytic metastases were overlooked on PET interpretation.
Three examples of malignant lesions at the sternum on 18F-fluoride PET/CT, with CT images shown in the left column, 18F-fluoride PET images in the middle, and fused PET/CT images on the right. Top row, images of a patient with breast cancer, shows increased uptake in a metastasis that is seen on CT to be loss of bone marrow fat (arrows). Middle row, images of a patient with breast cancer, shows increased uptake in a metastasis that is seen on CT to have corresponding sclerotic changes (arrows). Bottom row, images of a patient with multiple myeloma, shows increased uptake at the margin of a lytic lesion (arrows) that is seen on CT. The normal bone is replaced by a soft-tissue mass (arrowhead).
Three examples of malignant lesions at the vertebral column on 18F-fluoride PET/CT, with CT images shown in the left column, 18F-fluoride PET images in the middle, and fused PET/CT images on the right. Top row, images of a patient with breast cancer, shows increased uptake in the left posterior elements of a metastasis for which evidence of sclerotic changes is seen on CT (arrows). The middle and bottom rows are images of vertebral malignant lesions in a patient with multiple myeloma. In the middle row, increased uptake is seen at the margin of a lytic lesion (arrows). In the bottom row, CT shows a soft-tissue mass replacing normal bone (arrowhead), and increased uptake is detected at the margins of the lytic lesion (arrows).
For 85 of the 89 benign sites of increased 18F-fluoride uptake (96%), the CT images showed a benign abnormality exactly at the corresponding location. Table 1 summarizes abnormalities identified as benign on fused PET/CT images but associated with increased 18F-fluoride uptake (Fig. 3). When the diagnostic accuracy of 18F-fluoride PET and PET/CT in differentiating benign from malignant bone lesions was compared in a lesion-based analysis, the sensitivity was 72% and 85%, respectively (χ2 value = 5.28; P < 0.05) if metastases categorized as inconclusive (i.e., benign could not be differentiated from malignant) were considered false negative, and 90% and 99%, respectively, if they were considered true positive. The specificity of PET and PET/CT in differentiating benign from malignant lesions was 72% and 97%, respectively (χ2 value = 18.38; P < 0.001).
Two examples of benign bone abnormalities identified on 18F-fluoride PET/CT. Columns from left to right show maximum-intensity-projection image, CT, PET, and fused PET/CT images. Top row shows a subchondral bone cyst at the femur in a patient with prostate cancer. Bottom row shows an enchondroma at the right middle condyle of the femur in a patient with melanoma.
Benign CT Findings Identified on PET/CT in a Corresponding Location with Increased 18F-Fluoride Uptake
In 18 of the 200 lesions with a known final diagnosis, no CT abnormality was identified in a location corresponding to the increased 18F-fluoride uptake. Sixteen lesions (89%) were found to be metastases based on correlation with MRI (n = 3), with 18F-FDG PET/CT (n = 6), or with clinical and imaging follow-up (n = 7). A diagnostic full-dose CT study, which was available for correlation for 11 of those 16 lesions, also had negative results. When increased 18F-fluoride uptake and normal CT findings were considered to be an additional pattern suggestive of metastases on PET/CT assessment, the sensitivity increased to 99%. Table 2 summarizes the comparison of PET and PET/CT imaging in differentiating benign from malignant sites of 18F-fluoride uptake at various skeletal regions.
Comparison of 18F-Fluoride PET and PET/CT Results in Assessment of Metastatic Bone Disease
Patient-Based Analysis
Of the 44 study patients, 26 had evidence of skeletal tumor involvement: 20 with multiple tumor sites and 6 with a solitary lesion. Among the 18 patients with no evidence of malignant bone involvement, 2 had negative 18F-fluoride findings and 16 had increased uptake in benign bone lesions. When the diagnostic accuracy of PET and PET/CT in the assessment of skeletal tumor involvement in a patient-based analysis was compared, the sensitivity was 88% and 100%, respectively (χ2 value = 5.33; P < 0.05) and the specificity was 56% and 88%, respectively (χ2 value = 3.2; P = not statistically significant).
Of the 26 patients with skeletal tumor involvement, PET/CT was true positive in all patients and PET was true positive in 23 patients. The 3 patients with false-negative PET findings included a patient with breast cancer and metastases at the thoracic cage (lesions interpreted on PET as fractures), a patient with prostate cancer and metastases at the vertebral column (lesions interpreted on PET as degenerative changes), and a patient with lymphoma and bone involvement at the iliac wing and sacroiliac region (lesions interpreted on PET as an avulsion injury and inflammatory joint changes, respectively). The full extent of malignant bone involvement suggested on PET/CT images was seen on PET images for only 12 of the 23 patients with true-positive PET findings. In the remaining 9 patients (48%), although PET accurately revealed the presence of malignant involvement in some of the bone lesions, the findings were false negative at other malignant sites, which were categorized as benign or inconclusive. Of the 18 patients without bone involvement, the PET findings for 7 were false positive. In 2 of those 7, the PET/CT findings were also false positive, with radionecrosis and postsurgical changes being misinterpreted as tumor sites.
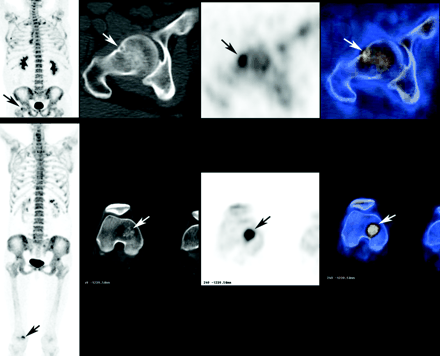
Twelve of the study patients were referred for 18F-fluoride PET/CT because of bone pain despite normal findings on bone scintigraphy. Four of the 12 patients had bone metastases that were accurately diagnosed using PET/CT, including a patient with a gastrointestinal stromal tumor, a patient with multiple myeloma, a patient with a metastatic giant cell tumor of bone, and a patient with prostate cancer (Fig. 4). Excluding the metastatic lesions detected by 18F-fluoride PET/CT in the patient with prostate cancer, all remaining malignant bone lesions missed on bone scintigraphy were lytic. Seven of the 12 patients did not have evidence of bone metastases, and 1 patient had a soft-tissue tumor invading a sacral foramen without bony involvement. That abnormality had been suggested on the CT portion of the PET/CT examination, and the finding was validated by MRI. In 4 of the 7 patients without bone metastases, benign lesions were identified on PET/CT as a potential explanation for pain. These benign lesion included degenerative changes (n = 2) and fractures (n = 2: 1 colon cancer patient with an insufficiency fracture at the sacroiliac region after radiotherapy; 1 prostate cancer patient with a rib fracture). PET/CT findings were normal, with no evidence of either malignant or benign changes, in 2 additional patients and were inconclusive, with a subsequent histopathologic diagnosis of radionecrosis, in 1 patient.
Comparison of 99mTc-MDP bone scintigraphy and 18F-fluoride PET/CT performed 11 d apart on a patient with prostate cancer who was referred for imaging because of skeletal pain. (A) Anterior (left) and posterior (right) bone scintigraphy images show increased uptake at the lumbar spine. When correlated with CT findings, uptake was found to be caused by degenerative changes. (B) Maximum-intensity-projection 18F-fluoride PET image shows multiple sites of increased uptake. (C) 18F-Fluoride PET/CT images of the pelvis show increased uptake at the sacrum and at the right iliac wing (arrow and arrowhead, respectively), with corresponding changes on CT.
DISCUSSION
18F-Fluoride is a bone-imaging agent for PET. After diffusing into the extracellular fluid of bone, the fluoride ion is exchanged for a hydroxyl group in the bone crystal and forms fluoroapatite, which then deposits at the bone surface where turnover is greatest (3,4). Uptake of the fluoride ion (18F) is 2-fold higher than that of 99mTc-polyphosphonates. The combination of the better spatial resolution of PET and the improved image quality achieved by the favorable pharmacokinetic characteristics of 18F-fluoride has led to the use of 18F-fluoride PET in the evaluation of skeletal metastases. Increased uptake has been detected in both sclerotic metastases and lytic metastases, with a significant superiority of 18F-fluoride PET over planar or SPECT bone scintigraphy in the detection of both benign and malignant bone (7,8,16). The high sensitivity of 18F-fluoride is also true in nonmalignant bone pathologies. That fact, however, limits its specificity when differentiation between a malignant and a benign lesion is essential. As in the case of bone scintigraphy, lesions detected on 18F-fluoride PET often require correlation with CT or MRI for further validation (8). These data encouraged us to use integrated PET/CT technology, recently introduced into our routine practice, for 18F-fluoride assessment of malignant bone involvement.
The same number of sites with increased 18F-fluoride uptake was found on PET and PET/CT images, except for 2 lytic metastases—detected as increased uptake at the margins of the lytic zone—that were overlooked on PET interpretation. However, the sensitivity of PET/CT in differentiating malignant from benign bone lesions, both in a lesion-based analysis and in a patient-based analysis, was superior. Tumor lesions that were categorized as benign or inconclusive on PET images were accurately diagnosed as malignant on PET/CT when lytic, sclerotic, or intramedullary changes were found in the corresponding location on the CT images. Those changes were observed in 94 of the 111 malignant bone lesions (85%), obviating the performance of full-dose diagnostic CT for correlative purposes in addition to the low-dose CT of the PET/CT study.
In the initial analysis of results, lesions that were inconclusive on PET or PET/CT interpretation were categorized as false negative, and the calculated sensitivity of PET and PET/CT was 72% and 85%, respectively. Lesions presenting on PET/CT as sites of increased uptake with normal CT findings (showing neither benign nor malignant changes) were categorized on PET/CT interpretation as inconclusive. This strict approach was used because in clinical practice, an inconclusive scintigraphic finding cannot be treated as such and warrants further assessment by either imaging or biopsy. The additional effort is obviously worthwhile if the final diagnosis is a malignancy but can worry the patient unnecessarily if the final diagnosis is a benign lesion. Bone lesions with a PET/CT pattern of increased 18F-fluoride uptake but normal CT findings were shown, however, to be associated with a high malignancy rate (89%). When that pattern was included among the PET/CT patterns associated with malignancy, the sensitivity of PET/CT for detection of malignant bone involvement reached 99%.
In a patient-based analysis, PET suggested malignant bone involvement in 23 of the 26 (88%) patients with evidence of bone tumor sites, compared with 100% by PET/CT. However, in 48% of the patients, PET alone failed to identify the full extent of tumor sites detected by PET/CT.
The high sensitivity of 18F-fluoride PET is also illustrated by the variety of benign causes of increased 18F-fluoride uptake, among them lesions that are usually not detected by bone scintigraphy, such as uncomplicated small subchondral cysts (Fig. 3; Table 1). This high sensitivity of 18F-fluoride for both benign and malignant lesions may pose a diagnostic dilemma, and lesions should accurately be differentiated as benign or malignant. On the other hand, by identifying not only malignant but also benign potential causes for pain, this sensitive modality may be beneficial if pain was the indication for the study.
In a lesion-based analysis, the overall specificity in differentiating benign from malignant lesions was 72% when 18F-fluoride PET was interpreted alone. The overall specificity of PET/CT interpretation was 97%. Specificity in the spine increased from 86% for PET alone to 100% for PET/CT; in the thoracic cage, from 40% to 87%; in the pelvis, from 67% to 89%; in the skull and facial bones, from 30% to 100%; and in the long bones, from 25% to 100%.
Soft-tissue abnormalities may be identified incidentally on the CT images of PET/CT: Exploring this issue was beyond the scope of the study. We did observe, however, a soft-tissue pelvic mass that seemed to invade the adjacent sacral foramen in 1 of the 12 patients complaining of bone pain.
99mTc-MDP bone scintigraphy, 18F-FDG PET, and 18F-fluoride PET are different scintigraphic approaches for assessment of malignant bone involvement. Because the 3 radiopharmaceuticals differ in pharmacokinetic characteristics, they also differ in sensitivity for the detection of bone lesions in variable malignant disease. The population investigated has a major effect on the results of studies assessing the performance of each approach (4,10,17–23). Our study population was heterogeneous, with only a few patients in each disease category. Bone scintigraphy and 18F-FDG PET were only occasionally available for correlation. Thus, the study results provide no grounds to suggest any superiority of 18F-fluoride PET/CT over bone scintigraphy or over 18F-FDG PET, despite the high diagnostic accuracy found for 18F-fluoride PET/CT. A study with a more homogeneous population, a direct comparison of 18F-fluoride PET/CT with bone scintigraphy or 18F-FDG PET, and a cost-effective analysis is needed to identify a clinical situation or patient population that warrants replacing the more commonly used studies by 18F-fluoride PET/CT.
CONCLUSION
The study found that 18F-fluoride PET/CT is both sensitive and specific for the detection of lytic and sclerotic malignant bone lesions and is superior to 18F-fluoride PET. PET/CT accurately differentiated malignant from benign lesions and possibly helped identify a potential cause for bone pain in oncologic patients. For most lesions, the anatomic data provided by the low-dose CT portion of the PET/CT study obviates the performance of full-dose diagnostic CT for correlation purposes.
Footnotes
Received Aug. 29, 2003; revision accepted Oct. 14, 2003.
For correspondence or reprints contact: Einat Even-Sapir, MD, PhD, Department of Nuclear Medicine, Tel-Aviv Sourasky Medical Center, 6 Weizman St., Tel-Aviv, 64239 Israel.
E-mail: evensap{at}tasmc.health.gov.il