Abstract
The α7-nicotinic cholinergic receptor (α7-nAChR) is a key mediator of brain communication and has been implicated in a wide variety of central nervous system disorders. None of the currently available PET radioligands for α7-nAChR are suitable for quantitative PET imaging, mostly because of insufficient specific binding. The goal of this study was to evaluate the potential of 18F-ASEM (18F-JHU82132) as an α7-nAChR radioligand for PET. Methods: The inhibition binding assay and receptor functional properties of ASEM were assessed in vitro. The brain regional distribution of 18F-ASEM in baseline and blockade were evaluated in DISC1 mice (dissection) and baboons (PET). Results: ASEM is an antagonist for the α7-nAChR with high binding affinity (Ki = 0.3 nM). 18F-ASEM readily entered the baboon brain and specifically labeled α7-nAChR. The in vivo specific binding of 18F-ASEM in the brain regions enriched with α7-nAChRs was 80%–90%. SSR180711, an α7-nAChR–selective partial agonist, blocked 18F-ASEM binding in the baboon brain in a dose-dependent manner, suggesting that the binding of 18F-ASEM was mediated by α7-nAChRs and the radioligand was suitable for drug evaluation studies. In the baboon baseline studies, the brain regional volume of distribution (VT) values for 18F-ASEM were 23 (thalamus), 22 (insula), 18 (hippocampus), and 14 (cerebellum), whereas in the binding selectivity (blockade) scan, all regional VT values were reduced to less than 4. The range of regional binding potential values in the baboon brain was from 3.9 to 6.6. In vivo cerebral binding of 18F-ASEM and α7-nAChR expression in mutant DISC1 mice, a rodent model of schizophrenia, was significantly lower than in control animals, which is in agreement with previous postmortem human data. Conclusion: 18F-ASEM holds promise as a radiotracer with suitable imaging properties for quantification of α7-nAChR in the human brain.
Nicotinic cholinergic receptors (nAChRs) are ionotropic cationic channels that are fundamental to physiology and, as a class, represent an important target for drug discovery. nAChRs are found in the central nervous system (CNS), autonomic and sensory ganglia, and various nonneuronal cells. In the CNS, nAChRs mediate fast excitatory postsynaptic responses to its cognate ligand acetylcholine and other nicotinic agonists (1).
Cerebral nAChRs are composed of various α and β subunits that can assemble into pentamers, with α4β2-nAChR and α7-nAChR subtypes representing the highest concentration of nAChRs in the CNS (2). The α7-nAChR is involved in pathogenesis of a variety of disorders and conditions including schizophrenia and Alzheimer disease, inflammation and traumatic brain injury, cancer, and macrophage chemotaxis (3–8).
Despite intense study, the role of α7-nAChRs in the brain is not fully understood. PET is the most advanced technique to map and quantify cerebral receptors and their occupancy by neurotransmitters and drugs in human subjects. The lack of PET radioligands for quantitative imaging of the α7-nAChRs in human subjects represents a gap that hampers research of this receptor system and development of new drugs for this target.
Over the past decade, considerable effort has been expended toward the development of α7-nAChR ligands, and more than 20 compounds have been radiolabeled for PET and SPECT, but previous efforts by several research groups (9–11), including our own (12–14), to develop a clinically viable α7-nAChR tracer for PET or SPECT have proved unsuccessful. None of these radioligands manifested sufficiently high specific binding at α7-nAChRs in vivo. Even 11C-CHIBA-1001, the recent PET radioligand for human subjects, exhibited a low α7-nAChR binding affinity and poor in vivo selectivity (15,16). Accordingly, there is still a pressing scientific need for a practical PET radiotracer for quantification of α7-nAChRs.
As part of our PET radioligand development program, we developed 18F-ASEM (18F-JHU82132, 3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-6-[18F]fluorodibenzo[b,d]thiophene 5,5-dioxide), an 18F-labeled, high-affinity and -selectivity α7-nAChR PET radioligand (Fig. 1) that showed excellent specific binding (BPND = 8) in control CD-1 mice (17). Here, we describe further preclinical characterization of 18F-ASEM in vitro and in vivo in baboon PET imaging studies and in DISC1 mice, a rodent model of schizophrenia.
Chemical structure of high-binding-affinity α7-nAChR–selective PET radioligand 18F-ASEM (18F-JHU82132) (17).
MATERIALS AND METHODS
All experimental animal protocols were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions.
18F-ASEM and unlabeled ASEM were synthesized as described by our laboratory (17). Briefly, the radiotracer was prepared by nucleophilic radiofluorination of the corresponding nitro precursor. The final product was purified by high-performance liquid chromatography (HPLC) and formulated as a sterile, apyrogenic solution in saline containing 8% alcohol. The specific radioactivity of the tracer was in the range of 296–2,180 GBq/μmol (8–59 Ci/μmol), calculated at the end of synthesis, and the radiochemical purity was greater than 99%. The average radiochemical yield was 15% ± 7% (n = 12).
In Vitro Inhibition Binding Assay of ASEM and Functional Electrophysiology Method
HEK293 cell culture and stable transfections of α7-nAChR and the ASEM inhibition binding assay with 125I-α-bungarotoxin were performed as described previously (supplemental data; supplemental materials are available at http://jnm.snmjournals.org) (18).
Whole-cell voltage clamp (holding potential, −70 mV) recordings from HEK293 cells stably transfecting the rat α7‐nAChR were made with patch electrodes (5–6 MΩ) containing a solution (pH 7.2) composed of potassium gluconate (145 mM), ethylene glycol tetraacetic acid (5 mM), MgCl2 (2.5 mM), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (10 mM), adenosine triphosphate sodium (ATP.Na) (5 mM), and guanosine triphosphate sodium (GTP.Na) (0.2 mM). Cells were continuously perfused with recording solution with the following composition: NaCl (130 mM), KCl (5 mM), CaCl2 (2 mM), MgCl2 (2 mM), glucose (10 mM), and HEPES (10 mM), pH 7.4, at a temperature of 24°C. The patch pipette was coupled to an amplifier (Axopatch 200B; Molecular Devices) and its signal filtered (5 kHz), digitized with a Digidata 1440A (Molecular Devices), and analyzed with pClamp 10 software (Molecular Devices). Acetylcholine was delivered to the cells rapidly by pressure application (picospritzer; World Precision Instruments) for 0.5 s. A bath was applied to the compound ASEM for 2 min before and during the application of acetylcholine by pressure application.
Biodistribution Study in Mutant DISC1 and Control Mice
Male DISC1 (16–18 g) and control (17–19 g) mice both on a C57BL/6 background were generated as previously described (19) and were used for biodistribution studies, with 6 animals per data point. The animals were sacrificed by cervical dislocation at 90 min after injection of 18F-ASEM (2.6 MBq; specific radioactivity, ∼300 GBq/μmol, in 0.2 mL of saline) into a lateral tail vein. The brains were rapidly removed and dissected on ice. The brain regions of interest were weighed, and their radioactivity content was determined in an automated γ counter with a counting error below 3%. Aliquots of the injectate were prepared as standards, and their radioactivity content was determined along with the tissue samples. The percentage injected dose per gram of tissue (%ID/g tissue) was calculated.
Western Blot with DISC1 and Control Mice
Mice were euthanized at postnatal day 21 to evaluate the expression of α7-nAChR in mutant DISC1 and control animals. Frontal cortices were quickly dissected out on ice-cold phosphate-buffered saline and frozen on dry ice and kept at −80°C until used. These samples were assayed for expression of mutant DISC1 (19). Membranes were incubated overnight at 4°C with either mouse anti-myc antibody (Santa Cruz Biotechnology Inc.; 1:1,000) to assess the expression of mutant DISC1 tagged with myc or rabbit polyclonal antibody to α7-nAChR (ab10096 [Abcam Inc.]; 1:500). Secondary antibodies were peroxidase-conjugated goat antimouse (Kierkegaard Perry Labs; 1:1,000) or sheep antirabbit (GE Healthcare; 1:2,500). The optical density of protein bands on each digitized image was normalized to the optical density of β-tubulin as a loading control (Cell Signaling Technology Inc; 1:10,000). Normalized values were used for statistical analyses.
Baboon PET Imaging and Baboon PET Data Analysis
PET experiments were performed on 3 male baboons (Papio anubis; weight, 20.1–26 kg) on the High Resolution Research Tomograph (CPS Innovations, Inc.). The animals were anesthetized and handled as described previously (supplemental data) (20). Three animals were scanned with 18F-ASEM in baseline scans. Dynamic PET images were acquired in a 3-dimensional list-mode for 90 min after an intravenous bolus injection of 18F-ASEM (246–319 MBq; n = 3), with specific radioactivities in the range of 343–1,764 GBq/μmol. PET images were reconstructed as described in the supplemental data.
In 2 blocking scans, the blocker SSR180711 solution in saline was given as intravenous bolus doses (0.5 or 5 mg/kg) 90 min before the radioligand 18F-ASEM injection (doses, 147 and 251 MBq; specific radioactivity, 462 and 1,014 GBq/μmol). The blocking scans were obtained for 1 of the baboons that were used in the baseline scans and separated at least 32 d from each other and the baseline scan.
A locally developed volume-of-interest (VOI) template was transferred to each animal’s MR image using spatial normalization parameters given by SPM5 (statistical parametric mapping (21); available at http://www.fil.ion.ucl.ac.uk/spm/software/spm5) and adjusted for anatomic details. Then, VOIs were transferred to the PET spaces of the baseline and blocking scans using the MR imaging–to–PET coregistration module of SPM5 (22). Time–radioactivity curves (time–activity curves) of regions were obtained by applying the VOIs on PET frames.
One- and 2-tissue-compartmental models (TTCM) were used for derivation of regional distribution volume (VT) for 18F-ASEM, with and without setting the K1–k2 ratio to the estimate of a large region (denoted as TTCM-C). Akaike information criteria (23) and the numbers of outliers were used to identify the optimal plasma input method for the radioligand. In addition, the plasma reference graphical analysis (PRGA) was evaluated (24). In blocking scans, occupancies of α7-nAChRs by SSR180711 were obtained as follows: occupancy = ΔVT/(VT[baseline] − VND), where ΔVT was given by VT(baseline) − VT(blocking), and VND, the distribution volume of nondisplaceable radioligand, was obtained as the x-intercept of the Lassen plot (25) of ΔVT (=y) versus baseline VT.
18F-ASEM: Radiometabolite Analysis in Baboon and Mice
Baboon arterial blood samples were withdrawn at 5, 10, 20, 30, 60, and 90 min after 18F-ASEM injection, and plasma was analyzed by HPLC. Male CD-1 mice (25–26 g) were injected via the lateral tail veins with 37 MBq of high-specific-activity 18F-ASEM. The mice were killed by cervical dislocation at 2 and 30 min after injection, and a terminal blood sample was obtained. The mouse brains were rapidly removed and analyzed by HPLC (supplemental data).
RESULTS
Binding Affinity
In 2 experiments, unlabeled ASEM exhibited high in vitro binding affinity to HEK293 cells stably transfected with rat α7-nAChR (Ki = 0.3, 0.3 nM) (125I-α-bungarotoxin).
In Vitro Functional Assay
The functional activity of unlabeled ASEM was examined using whole-cell voltage clamp measurements in HEK293 cells expressing α7-nAChRs. As shown in Figure 2, acetylcholine at a concentration of 316 μM activates these receptors, and ASEM at a concentration of 1 nM nearly completely blocked activation by acetylcholine. Moreover, a partial block persists during the short period of washing, probably because of the high affinity of ASEM.
Unlabeled compound ASEM (JHU82132) inhibits activation of acetylcholine-stimulated rat α7-nAChRs. Whole-cell voltage clamp current activated with 316 μM acetylcholine either before or during bath application of 1 nM ASEM was determined in HEK293 cells stably transfected with rat α7-nAChRs. Bath application of ASEM for 2 min before and during application of acetylcholine inhibited subsequent acetylcholine-induced whole-cell current. This current was restored to 60% of baseline after 12 min of washing. ACh = acetylcholine.
Brain Distribution of 18F-ASEM in Mutant DISC1 and Control Mice
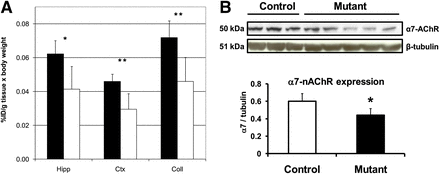
Mutant DISC1 mice provide a model for brain and behavioral phenotypes seen in schizophrenia (19). The comparison of regional brain uptake of 18F-ASEM in mutant DISC1 versus control mice demonstrated that the uptake in the mutant mice was significantly lower in all regions studied. Because of the difference in the mouse weight (up to 15%), the uptake values were corrected for the body weight (%ID/g tissue × body weight) (Fig. 3A).
(A) Comparison of regional uptake of 18F-ASEM in control (black bars) and DISC1 (white bars) mice at 90 min after injection. There was significant reduction of 18F-ASEM in DISC1 in brain regions studied. Data are mean %ID/g tissue × body weight ± SD (n = 6). *P = 0.01 and **P < 0.01, significantly different from controls (ANOVA). (B) Western blot. Expression of α7-nAChR protein in P21 cortex of mutant DISC1 (n = 5) is significantly lower than in that of control mice (n = 3). *P = 0.035 (Student t test, t = 2.7). Coll = superior and inferior colliculus; Ctx = cortex; Hipp = hippocampus.
Western blot analysis of the expression of α7-nAChR in the cortical regions was in agreement with the biodistribution of 18F-ASEM. We found a significant decrease in the levels of the receptor in the cortex of mutant DISC1 mice, compared with control mice (Fig. 3B).
PET Imaging in Papio Anubis Baboons
Heterogeneous uptake of radioactivity into the baboon brain was observed in baseline experiments after bolus injection of 18F-ASEM in 3 baboons (Figs. 4 and 5). The highest accumulation of radioactivity occurred in the thalamus, insula, and anterior cingulate cortex. The intermediate uptake was observed in the putamen, hippocampus, and several cortical regions. The lowest uptake was in the corpus callosum, pons, and cerebellum. The time–activity curves of the cerebellum peaked before 20 min and decreased rapidly, whereas time–activity curves of other regions were slower with wider peaks and decreased relatively slowly (Fig. 4). In the 3 baseline experiments, no blocking effect was observed due to the variation of 18F-ASEM specific activity from high (343 GBq/μmol) to very high (1,764 GBq/μmol).
Baseline cerebral time–activity curves after bolus administration of 18F-ASEM in 3 baboons. Graph demonstrates substantial heterogeneous brain uptake of 18F-ASEM that matches distribution of α7-nAChR in nonhuman primates (29,30,32) and reversible brain kinetics. Data are mean SUV (%SUV) ± SD (n = 3). aCg = anterior cingulate cortex; CB = cerebellum; CC = corpus callosum; Hp = hippocampus; In = insula; Oc = occipital lobe; Pa = parietal lobe; Po = pons; Pu = putamen; Th = thalamus; Tp = temporal lobe.
Averaged transaxial %SUV PET images (10–90 min) of 18F-ASEM (upper) at levels showing putamen (Pu/+), thalamus (Th/+), and cortices such as frontal (Fr/+) and parietal (Pa/x), as shown on MR images (lower). SUV = standardized uptake value.
The kinetics of 18F-ASEM in the brain fitted well to a TTCM. The PRGA plots reached an asymptote (the coefficient of determination, R2 > 0.995) at 30 min in all regions. Therefore, PRGA was used for further analyses. Regional values of VT of 18F-ASEM in baboon are shown in Figure 6. The thalamus, insula, and anterior cingulate cortex provided the highest VT values, and the pons, corpus callosum, and cerebellum showed the lowest VT values.
(A) Lassen plot for dose experiment of 5 mg/kg demonstrates that specific binding of 18F-ASEM is blocked by α7-nAChR–selective ligand SSR180711. Data points showed linear appearance (ΔVT = 0.82⋅VT − 0.66; R2 = 0.979; VND = 0.8 mL/mL). VND is given as x-intercept in plot. (B) Histogram of VT values of 18F-ASEM (PRGA) in selected brain regions of 1 baboon at baseline and after administration of 2 different doses of SSR180711. Graph demonstrates that regional binding of 18F-ASEM is specific and high and mediated by α7-nAChR. aCg = anterior cingulate cortex; Cb = cerebellum; CC = corpus callosum; Hp = hippocampus; In = insula; Oc = occipital lobe; Pa = parietal lobe; Po = pons; Pu = putamen; Th = thalamus.
Injection of SSR180771, a selective α7-nAChR partial agonist (Ki = 22 nM) (26), reduced the regional uptake of 18F-ASEM in the baboon brain in a dose-dependent manner (Fig. 7). Regional VT values in baseline and blockade experiments are shown in Figure 6.
Sagittal (top) and transaxial (middle and bottom) views of VT images of 18F-ASEM in same baboon for baseline PET scan (B) and after administration of 0.5 mg/kg (C) and 5 mg/kg (D) of SSR180711, a selective α7-nAChR partial agonist. MR images (A) indicate locations of selected brain structures including cingulate cortex (Cg), thalamus (Th), and caudate nucleus (CN), which are indicated by + in VT images (D). VT images were displayed using same minimum and maximum values for all scanning conditions. These data demonstrate dose-dependent blockade of 18F-ASEM in baboon brain and provide evidence that 18F-ASEM is specific and mediated by α7-nAChR. Images also suggest that there is no reference region devoid of α7-nAChRs.
Lassen plots showed a linear arrangement for 0.5 and 5 mg/kg doses, as exemplified for the dose of 5 mg/kg in Figure 6A (for a dose of 0.5 mg/kg, ΔVT = 0.39⋅VT − 2.1; R2 = 0.643; VND = 5.4 mL/mL). Mean occupancy values increased from 38% with a dose of 0.5 mg/kg to 80.5% with a dose of 5 mg/kg using individual VND values, and from 32.9% to 94.1% using the mean VND value of 2 doses. Although estimates of VND differed between 2 blocking scans, individual values were severalfolds lower than the lowest observed VT (14 mL/mL in the pons) among the tested regions. This finding confirmed the lack of α7-nAChR–free regions in the baboon brain and low nonspecific binding of 18F-ASEM across regions (e.g., less than 30% in the pons and cerebellum and lower in other regions) and explained consistent occupancy estimates. Regional BPND ([VT/VND] – 1) values of 18F-ASEM in the baboon brain ranged from 3.9 to 6.6 (unitless), using the mean VND value of the 2 blocking scans.
Metabolism of 18F-ASEM in Mouse and Baboon
Radiometabolite analysis of blood samples from CD-1 mice and baboons by reversed-phase HPLC showed that the parent compound 18F-ASEM was metabolized to 2 major hydrophilic species. The combined radiometabolites in the plasma reached values of 70% in baboons and approximately 99% in mice at 90 and 30 min after injection, respectively (supplemental data; Supplemental Fig. 1). These radiometabolites do not enter the brain to an appreciable extent, because at least 95% of the unchanged parent 18F-ASEM was present in the mouse brain versus approximately 1% in the mouse blood after intravenous administration of 18F-ASEM. The amount of unchanged parent 18F-ASEM in the baboon brain should be even greater than that in mouse (>95%) because the metabolism in baboon is slower. This observation suggests that modeling of the metabolites may not be necessary for quantification of α7-nAChR with 18F-ASEM.
DISCUSSION
Our previous in vitro binding assay studies demonstrated that ASEM exhibits high α7-nAChR binding affinity in rat brain membranes and excellent selectivity versus other heteromeric nAChR subtypes and 5-HT3 (17). Those studies demonstrated that ASEM exhibits at least an order of magnitude greater binding affinity than previous α7-nAChR PET radioligands (17). In this report, we have confirmed the high α7-nAChR binding affinity of ASEM in the binding assay with the HEK293 cell line expressing rat α7-nAChR (Ki = 0.3 nM).
The functional assay demonstrated that ASEM is a powerful antagonist of α7-nAChR (Fig. 2), which is in accord with functional properties of des-fluoro-ASEM, 3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)dibenzo[b,d]thiophene 5,5-dioxide, which was recently published by Abbott Labs (27). This functional property may also be advantageous from the standpoint of safety if 18F-ASEM is used in human PET studies because it should not cause toxic effects that are common among nicotinic agonists (28).
The initial in vivo distribution studies in control mice have demonstrated that 18F-ASEM selectively labels α7-nAChR with very high specificity (BPND = 8) (17). On the basis of the favorable imaging properties identified in normal mice, we investigated 18F-ASEM cerebral binding in mutant DISC1 mice, a rodent model of schizophrenia (19). Previous postmortem research demonstrated significantly lower densities of α7-nAChR in the cortical and subcortical (hippocampus) brain regions of schizophrenic subjects versus controls (8). In agreement with this in vitro human data, the brain regional distribution experiments with DISC1 mice showed a significant reduction of 18F-ASEM binding in the α7-nAChR–rich colliculus, cortex, and hippocampus in comparison with control animals (Fig. 3A). Western blot data (Fig. 3B) of α7-nAChR protein expression in the cortex of DISC1 and control animals was in agreement with 18F-ASEM binding.
This result in DISC1 mice is consistent with previous postmortem brain studies of subjects with schizophrenia (8) and further emphasizes the potential utility of this new radioligand for imaging α7-nAChR in disease.
18F-ASEM exhibited high (500 % standardized uptake value [SUV]) and reversible brain uptake in baboon brain experiments (Figs. 4 and 5). The cerebral α7-nAChR is heterogeneously distributed in the primate brain, with the highest concentration in the thalamus, putamen, several cortical regions, and hippocampus (29–32). The observed PET regional distribution patterns of 18F-ASEM in the baboon brain (thalamus > putamen, cortex, hippocampus > caudate nucleus, globus pallidus > corpus callosum) are consistent with in vitro data in rhesus and cynomolgus macaque monkeys (29,30,32). The existing quantitative nonhuman primate data describing the brain distribution of α7-nAChR using in vitro autoradiography are detailed only for subcortical regions but limited for cortical regions or semiquantitative (29,30,32). The PET 18F-ASEM baboon experiments demonstrated that the lowest α7-nAChR uptake, albeit still considerable, was in the cerebellum. The cerebellum was not assessed in the previous monkey autoradiography studies (29,30,32). It is noteworthy that the uptake of radioactivity in the baboon skull was low, suggesting little metabolism of 18F-ASEM to 18F-fluoride that can confound PET studies with 18F-labeled agents.
The dose-dependent blockade of 18F-ASEM with the selective α7-nAChR partial agonist SSR180711 (Figs. 6 and 7) demonstrated that the binding of the radioligand in the baboon brain was specific (up to 80%–90%) and mediated by α7-nAChR. The level of specific binding of 18F-ASEM is well above the conventional minimum of the required specific binding value (≥50%) for a clinically viable PET radioligand. 18F-ASEM is suitable for quantitative analysis, and its BPND values (3.9–6.6) in the baboon brain are rather high. For comparison, the BPND values of all previously published α7-nAChR radioligands did not exceed 1 (9–11,17). This high specific binding of 18F-ASEM in combination with high brain uptake and VT values, reversible brain kinetics, and absence of active metabolites make this radioligand an excellent candidate for further translation to human PET imaging of α7-nAChRs.
CONCLUSION
We have developed a new specific α7-nAChR ligand, 18F-ASEM, that demonstrates suitable properties for imaging this important CNS target with PET in mice and baboons. Unlike its predecessors, 18F-ASEM has proved to be amenable to quantitative analysis with a useful degree of binding specificity (up to 80%–90%) and high BPND values of 3.9–6.6 in the α7-nAChR–rich brain regions as demonstrated by in vivo receptor blockade studies in baboons.
The brain uptake of 18F-ASEM in an established rodent model of schizophrenia, DISC1 mutant mice, reflects reduced α7-nAChR binding, which has been shown previously only in postmortem human studies, lending further support to this model and suggesting the high utility of 18F-ASEM for studying diseases of nicotinic transmission.
The 18F-ASEM radioligand holds considerable promise for human studies for understanding the role of α7-nAChRs in CNS disorders and will aid α7-nAChR–targeted drug development. Experiments in relevant human populations are being vigorously pursued.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This research was supported by NIH grants MH079017 and AG037298 and, in part, by the Division of Nuclear Medicine of Johns Hopkins University School of Medicine. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Dr. Richard Wahl for fruitful discussions. We also thank Drs. Weiguo Ye, Asifa Zaidi, and Jennifer Coughlin for injecting baboons with 18F-ASEM. We are grateful to Paige Finley, Heather Valentine, Gilbert Green, and Chunxia Yang for their valuable help with baboon and mouse experiments; David J. Clough and Karen Edmonds for PET scanner operation; and Alimamy Kargbo for HPLC analysis of radiometabolites. We are thankful to Julia Buchanan for editorial help.
Footnotes
Published online Feb. 20, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication September 3, 2013.
- Accepted for publication December 2, 2013.