Abstract
The purpose of this study was to determine the diagnostic value of 131I SPECT/spiral CT (SPECT/CT) on nodal staging of patients with thyroid carcinoma at the first ablative radioiodine therapy. Methods: Fifty-seven patients were studied using SPECT/CT 3–4 d after receiving 3.96 ± 0.5 GBq of 131I for radioablation of thyroid remnants after a thyroidectomy for differentiated thyroid carcinoma. In addition to planar whole-body scintigraphy, SPECT/CT of the neck was performed using a hybrid camera combining a double-head SPECT camera with either a 2-slice (n = 23) or a 6-slice (n = 34) spiral CT scanner. The planar scans and the SPECT/CT images were evaluated for cervical tracer uptake independently of each other and of the clinical findings. Results: SPECT/CT led to a revision of the original diagnosis in 28 of 143 cervical foci of radioiodine uptake seen on planar imaging. In particular, SPECT/CT reclassified as benign 6 of 11 lesions considered to be lymph node metastases and 11 of 15 lesions considered to be indeterminate. Furthermore, SPECT/CT allowed the identification of 11 lymph node metastases classified as thyroid remnant or as indeterminate on planar imaging. Based on this revision, SPECT/CT yielded a gain in information on nodal stage in 20 of the 57 patients studied (35%, P < 0.03). SPECT/CT altered nodal stage from N0 to N1 in 2 of 20 patients and from indeterminate (Nx) to N1 in 6 of 30 patients. The result was a change in risk stratification conforming to the classification proposed by the International Union Against Cancer in 14 patients (25%). Conclusion: SPECT/CT determines lymph node involvement at radioablation performed for thyroid cancer more accurately than does planar imaging. SPECT/CT may alter management in roughly one quarter of patients with thyroid carcinoma by upstaging or downstaging their disease.
With the exception of unifocal microcarcinoma with no extension beyond the thyroid capsule and without lymph node metastases (LNMs), the standard treatment of differentiated thyroid carcinoma (DTC) includes total thyroidectomy and therapy with 131I-iodide (1,2). With this combined approach, 5-y survival rates exceed 90% overall.
The frequency and type of follow-up after initial therapy are still under discussion (3–5). Current guidelines recommend risk-adapted patient management (6). In the cancer TNM staging system developed by the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) (7), the demonstration or exclusion of metastases in the regional lymph nodes plays a major role since all patients with LNMs are attributed to the high-risk group.
LNM can be detected by elective neck dissection. However, elective neck dissection is not performed in a significant number of patients with DTC (6,8,9). Furthermore, in a minority of patients, LNM may elude surgical removal (10).
Scintigraphy performed after radioiodine ablation also offers the possibility of nodal staging. However, the high activity contained in the thyroid residue may hamper the detection of radioiodine-positive cervical lymph nodes. Moreover, precise evaluation of cervical foci of 131I uptake as benign residual thyroid tissue or LNM can be difficult because of the poor anatomic information provided by planar 131I scintigraphy. Both of these reservations apply to SPECT as well.
Hybrid cameras combining a dual-head SPECT camera with a CT scanner in one gantry have now been commercially available for some time (11). By relating foci of 131I uptake to CT morphology, hybrid cameras may have the potential to overcome at least some of the shortcomings of 131I scintigraphy.
In our department, SPECT/CT has been routinely implemented since 2006 in the posttherapeutic imaging protocol for patients with DTC after the first radioablation. The aim of the present study was to compare the diagnostic value of 131I SPECT/spiral CT (SPECT/CT) with that of planar scintigraphy in the nodal staging of DTC at radioablation.
MATERIALS AND METHODS
Selection of Patients
From January 2006 until October 2007, 65 patients were admitted to the Clinic of Nuclear Medicine of the University of Erlangen/Nürnberg for their first radioablation of DTC 3–6 wk after thyroidectomy. In 54 of these patients, posttherapeutic SPECT/CT was performed. In 11 patients, SPECT/CT was not available because of technical or logistic reasons.
In addition to this group of patients, 3 patients studied in 2005 with SPECT/CT at radioablation were added.
The group thus selected comprised 22 men and 35 women. The ages ranged from 13 to 86 y (mean, 49 ± 15.45 y). All patients had histologically confirmed DTC (52 papillary, 4 follicular, and 1 papillary and follicular). Lymph node dissection was performed in 28 patients (median, 4 lymph nodes resected; range, 0–48). The postoperative staging of the primary tumor according to the AJCC/UICC TNM definition (8) and stage grouping reflecting risk stratification conforming to the AJCC/UICC were as described in Table 1.
Tumor Stage and TNM Classification as Defined by AJCC/UICC in the Patients Studied
Data Acquisition
In 54 patients, pretherapeutic thyroid-stimulating hormone levels were increased because of the absence of thyrosubstitution. The remaining 3 patients had intramuscular injections of recombinant thyroid-stimulating hormone 48 and 24 h before administration of radioiodine. All patients were prepared by restricting dietary iodine for at least 2 wk. Thyroid uptake was measured 24 h after application of a capsule containing 4–5 MBq of 131I to exclude large residual thyroid tissue. For the therapy, after 4 h of fasting, the patients received on average 3,957 MBq (range, 1,511–5,300 MBq) of 131I orally administered as a capsule.
Planar whole-body scintigraphy and separate imaging of the neck were performed 3.7 d (range, 2–7 d) after therapy, usually after a dose rate lower than 13 μSv/h at a 1-m distance. Immediately after planar scintigraphy, a SPECT/CT scan was acquired using a hybrid camera combining a dual-head γ-camera with a dual-slice (n = 23) or 6-slice (n = 34) spiral CT camera installed within the same gantry (Symbia T2 or T6, respectively; Siemens Medical Solutions USA, Inc.). The planar scintigraphy was acquired using the SPECT component of these hybrid cameras or a stand-alone dual-head γ-camera (Basicam; Siemens Medical Solutions). All cameras used were equipped with 0.9525-cm (3/8-in) NaI crystals.
All patients underwent the routine planar imaging protocol in our clinic, including whole-body scans and spot planar images of the cervical and abdominal regions. Whole-body scans were performed, acquiring both anterior and posterior images at a speed of 5 cm/min using high-energy, medium-sensitivity parallel-hole collimators, a 512 × 512 matrix, and a 364-keV photopeak with 15% windows. In addition, anterior and posterior images of the cervical and thoracic regions and of the abdomen were acquired at a rate of 5 min per bed position using high-energy, medium-sensitivity parallel-hole collimators, a 512 × 512 matrix, and a 364-keV photopeak with 15% windows.
The SPECT scan was obtained first. The counts from the 15% energy windows at 364 keV were acquired into a 64 × 64 matrix (pixel size, 4.6 × 4.6 mm). A total of 64 frames, each with a duration of 45 s, were acquired over 360°. The camera heads were equipped with high-energy, low-penetration parallel-hole collimators. The field of view of the SPECT camera included the cervical region/thyroid bed in the center. To reduce radiation exposure, the field of view of the CT camera was restricted to the area where the 131I-avid foci were located on the planar images (“guided CT”). For this aim, a CT topogram was acquired and the scan area for CT was planned using the anatomic information from the planar images. Thereafter, the CT scan was obtained. The scan parameters for CT were 130 kV, a rotation time of 0.6 s, and a 2 × 2.5 mm collimation. Because only cervical structures had to be analyzed, the tube current was reduced to 40 mAs to minimize radiation exposure.
Both SPECT and CT were acquired during shallow breathing. During both the SPECT and the CT scans, the patients were lying stably in a supine position. The interval between SPECT and CT was less than 3 min.
The SPECT data were reconstructed and displayed as transaxial, coronal, and sagittal slices using the syngo MI Applications software (Siemens Medical Solutions). Reconstruction was performed with Flash3D, the Siemens implementation of ordered-subsets expectation maximization using 3-dimensional isotropic resolution recovery with 4 iterations and 8 subsets. Images were smoothed with a 3-dimensional spatial gaussian filter with a full width at half maximum of 10 mm.
The CT data were reconstructed with a standard Feldkamp algorithm and the B40s kernel.
Image and Data Analysis
Visual analysis of the fused images disclosed no relevant misalignment between internal landmarks visible on both CT and SPECT, for example, the salivary glands. The planar and the SPECT/CT images were reviewed independently of the clinical data by 2 board-certified physicians, each with more than 10 y of experience in reading radioiodine scans. One of these two also had 2 y of experience reading CT.
On the screen of a dedicated workstation (syngo), all planar scans were evaluated for foci of uptake in the neck. First, foci of uptake interpreted as localized in the thyroid bed were identified; these were classified as thyroid remnants. Foci separable from uptake by these thyroid remnants were then classified. A focus of uptake in the midline above the thyroid bed was classified as pyramidal lobe; a focus close to the thyroid bed, as indeterminate; and a focus at a greater distance from the thyroid bed, as LNM.
On the SPECT/CT images, all foci with uptake greater than the background level were localized according to the schematic diagram indicating the cervical lymph node levels published by the AJCC in 2002 (8). In addition to the levels I–VII depicted in this scheme, we defined the region surrounded by the levels I, II, III, and VI, that is, the midline region between larynx and hyoid bone, as level VIII. These foci were then classified. A focus in level VI was classified as thyroid remnant; a focus in level VIII, as pyramidal lobe; a focus in levels I–V and VII colocated with lymph node visible on CT, as LNM; a focus in levels I–V and VII without lymph node visible on CT, as indeterminate; and a focus in the skin, as contamination.
For further analysis, foci classified as thyroid remnant or pyramidal lobe were grouped together under the heading of thyroid remnant.
RESULTS
Per-Lesion Analysis
On planar imaging, 143 cervical foci of radioiodine uptake were seen. No additional foci were disclosed by SPECT/CT. In 28 of these foci (19.5%), SPECT/CT led to a revision of the original diagnosis (Table 2). In particular, 6 of 11 lesions considered to be LNM and 11 of 15 lesions considered to be indeterminate were clearly reclassified as benign. One lesion classified as an LNM by planar imaging had to be reclassified as indeterminate since it was located outside level VI but had no morphologic counterpart. Furthermore, SPECT/CT allowed the identification of 11 LNMs misinterpreted as a thyroid remnant or classified as indeterminate on planar imaging. Typical patient examples are provided in Figures 1–3⇓⇓.
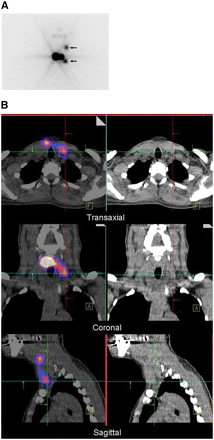
A 54-y-old woman with differentiated papillary thyroid carcinoma (pT2 N1a [6/21] Mx) after total thyroidectomy and lymph node dissection of centrocervical and left lateral compartment. (A) Planar scintigraphy shows two 131I-avid foci interpreted as thyroid remnant. (B) SPECT/CT (left column) and CT (right column) demonstrate that these foci correspond to LNMs in superior mediastinum (level VII)—shown here for right focus.
A 30-y-old woman with differentiated papillary thyroid carcinoma (pT2 N0 [0/10] Mx) after total thyroidectomy and lymph node dissection of centrocervical compartment. (A) Planar scintigraphy shows large 131I-avid foci interpreted as thyroid remnant and indeterminate. (B) SPECT/CT fusion images (left column) and CT (right column) demonstrate 1 focus corresponding to LNM in level II. (C) SPECT/CT (left column) and CT (right column) demonstrate 1 focus corresponding to LNM in level III.
A 43-y-old woman with differentiated papillary thyroid carcinoma (pT1 N0 [0/7] Mx) after total thyroidectomy and lymph node dissection of centrocervical compartment. (A) Planar scintigraphy shows 131I-avid foci interpreted as LNMs (arrows) and thyroid remnant. (B) SPECT/CT fusion images (left column) and CT (right column) demonstrate that these foci correspond to LNMs in level II and level IV—shown here for lower focus.
Planar Findings and SPECT/CT Characterization of Radioiodine-Positive Cervical Foci
Per-Patient Analysis
In 24 of 57 patients, the diagnosis reached by planar imaging was revised or specified by SPECT/CT. In 20 of 57 patients (35%, χ2 = 10.94, P < 0.03; Fig. 4), SPECT/CT yielded a gain in information on nodal stage. Compared with planar imaging, SPECT/CT resulted in a downstaging of disease in 12 patients. Upstaging occurred in 8 patients, with the nodal stage based on histopathologic diagnosis altered from N0 to N1 in 2 of 20 patients and from indeterminate (Nx) to N1 in 6 of 30 patients. The change of nodal stage resulted in a new risk stratification with consequences for planning follow-up in 14 of 57 (25%) patients: Nine patients were regrouped as low-risk and 5 patients as high-risk.
Imaging results in patients classified as N0 (A) or Nx (B) by histopathology. “Nx” denotes classification as indeterminate.
In one patient, the extrathyroidal focus of uptake diagnosed on the basis of planar scintigraphy could not be clarified by SPECT/CT. This patient then had to be reclassified as having Nx disease.
DISCUSSION
This paper reports the incremental diagnostic value of SPECT/CT over planar imaging performed at the first radioablation of thyroid remnants in 57 patients with DTC. Compared with planar imaging, SPECT/CT yielded a gain in information on nodal stage in 35% of the patients studied. Furthermore, SPECT/CT altered risk stratification in 25%, thus altering follow-up in these subjects.
The superiority of SPECT/CT over planar scintigraphy demonstrated in our patients agrees with previously published data gleaned from more heterogeneous patient groups (12,13).
The rationale for classifying foci of radioiodine uptake as cervical metastases on SPECT/CT images was their location outside lymph node levels VI and VIII, which contain the thyroid bed. We used the CT identifiability of a lymph node collocated with that focus as a further criterion; foci outside levels VI and VIII without structural correlates were considered indeterminate. This approach may underestimate the frequency of microscopic disease but potentially offers the advantage of higher specificity. Conversely, some of the foci at level VI might have been local recurrences or central LNMs. The first of these possibilities seems, however, highly improbable since most patients (49/57) had an R0 resection of their tumors; the second cannot be reliably ruled out. Therefore, also with SPECT/CT, the incidence of cervical LNMs may be underestimated in this patient population.
Clearly, a gold standard independent of SPECT/CT would be needed to further clarify this issue. This standard could, in principle, be either histology, clinical course, or results from other imaging modalities. Obviously, reoperation of all patients directly after the first radioablation to obtain histologic samples is not feasible. The assessment of clinical course is also not an option since radioiodine delivered at therapeutic doses may eliminate cervical LNMs. Other imaging modalities such as CT or MRI do not qualify as a gold standard in this patient group since they suffer from limited sensitivity and specificity, compared with radioiodine imaging.
Cervical ultrasound applying high-frequency probes is a widely used procedure to screen for LNM. However, small LNMs may escape detection by ultrasound although visible on 131I scintigrams. Enlarged lymph nodes could also represent postinflammatory lymph node hyperplasia, which is difficult to differentiate from LNM although some criteria for malignancy have recently been reported (14). The comparison between SPECT/CT and cervical ultrasound is—although worthwhile—beyond the scope of this publication. Nevertheless, a retrospective review of the patient charts disclosed that LNMs were identified in only 1 of 16 patients with a SPECT/CT diagnosis of LNM, illustrating that cervical ultrasound cannot serve as a gold standard in this patient group. Clearly, the distortion of anatomy by previous surgery might also explain the comparatively low sensitivity of ultrasound in our patient population.
From 2006 until 2007, 83% of all patients referred to our institution for radioablation were studied also by SPECT/CT. The reason for not examining the remaining 17% was the temporary unavailability of the hybrid camera or the unsuitability of the patient for this procedure, as would be the case in a patient of excessive body weight or who refuses to undergo SPECT/CT. The group we studied may therefore be considered representative of all patients referred to our hospital. Thirty-three percent of our patients had regional lymph node involvement diagnosed either histologically after lymph node dissection (12%) or by SPECT/CT (21%). This frequency is of the same magnitude as frequencies reported in the literature (15–18). However, because patient cohorts treated in other institutions may differ with regard to histology and tumor stage, our data should be extrapolated with some care.
Interestingly, SPECT/CT had the ability to detect lymph node involvement in patients staged as N0 after lymph node dissection. The number of lymph nodes resected ranged from 0 to 48 (median, 4) and might, at least in some subjects, not have been sufficient for reliable exclusion of nodal disease. In 2 subjects on whom only central dissection was performed, nodes in the lateral compartment ipsilateral to the primary tumor proved involved. A further explanation for this observation might be the occurrence of skip metastases leaping over the central lymph node compartment (10).
The data presented here suggest that SPECT/CT should be used as a routine procedure in DTC patients at the first radioablation. Nevertheless, some drawbacks of the present data should still be considered. SPECT/low-amperage CT adds a radiation dose ranging from 2 to 3 mSv to that of radioiodine. However, we believe that the hypothetical risk attributable to this dose is negligible compared with the obvious benefits of this hybrid imaging technology. Some further work will be needed to investigate whether subgroups of DTC patients might particularly benefit from SPECT/CT performed at radioablation. Our results should also motivate the creation of studies analyzing the impact of SPECT/CT on outcome variables such as patient survival or its cost efficiency—variables that were beyond the scope of the present publication.
CONCLUSION
SPECT/CT is more accurate than planar imaging for evaluating lymph node involvement at radioablation performed for thyroid cancer. SPECT/CT also allows detection of nodal spread in patients with disease classified as N0 by histologic evaluation of specimens from cervical lymph node dissection. By upstaging or downstaging disease, SPECT/CT may alter the management of roughly one quarter of patients with thyroid carcinoma.
Acknowledgments
The SPECT/CT camera Symbia T2, which was used to examine 23 of the 57 patients, was kindly provided by Siemens Medical Solutions, Erlangen, Germany. We gratefully acknowledge the technical support of Anja Reimann.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication March 20, 2008.
- Accepted for publication September 22, 2008.