Therapy with 90Y-microspheres is emerging as a mainstream treatment modality in the management of patients with primary and metastatic liver cancer (1,2). The technique involves the administration of 90Y-microspheres into the hepatic artery, which is accessed via the transfemoral route or through a hepatic arterial infusion port or pump. The technique was first introduced by Ariel, who also reported the first series of successful treatment in patients with metastatic colorectal cancer (3,4). The patients were treated with intraarterial chemotherapy and 3.7–5.5 GBq (100–150 mCi) of 90Y-resin microspheres. The estimated radiation dose to the liver from 3.7 GBq (100 mCi) of 90Y-microspheres was 120–180 Gy using the MIRD approach. It took a few decades since Ariel's early experience, in the 1960s, to refine the manufacturing technology and administration techniques of 90Y-microspheres before more structured studies could be implemented (5–7). The initial indications, for use in colorectal cancer metastases and hepatocellular carcinoma, have now expanded to include other unresectable metastatic liver tumors (8,9). The development of highly sophisticated techniques of administration has improved the therapeutic efficacy while minimizing the attendant side effects (10).
Compared with the growing clinical experience with 90Y-microsphere therapy, dosimetric data are unsatisfactory largely because of the lack of uniform and well-explained methods. This brief report aims to summarize the principles of 90Y-microsphere dosimetry and provide the mathematic derivations of the equations used in the MIRD schema.
Commercially available 90Y-microsphere products include resin microspheres with a specific activity of 40–70 Bq per sphere (SIR-Spheres; Sirtex Medical) and glass microspheres with a specific activity of 2,400–2,700 Bq per sphere (TheraSphere; MDS Nordion), both of which have median diameters of between 35 and 40 μm. Microspheres administered in the hepatic artery are distributed preferentially in the tumor compartment and are trapped within the microvasculature of the tumor. Microspheres are biocompatible but not biodegradable, and therefore no biologic elimination occurs. The entire 90Y dose is delivered over a physical decay period with a half-life of 2.66 d. Radiation delivery from 90Y-microspheres is essentially confined to the liver because of the 3.8-mm mean range and approximately 10-mm maximum range of β-particles in soft tissue.
Although, in reality, 90Y-microsphere distribution is never uniform and, in fact, is invariably patchy, with a wide range of variation, MIRD dose estimations are based on the assumption of a uniform distribution. Obviously, this assumption of uniform distribution of the microspheres is acceptable only as a first-order approximation. Despite this recognized limitation, the MIRD methodology provides consistent and reproducible dose estimates.
The MIRD Schema for 90Y-Microspheres
90Y-Microspheres are distributed in the liver parenchyma with a concentration of C μCi/g. Because 1 μCi produces 3.7 × 104 disintegrations per second, energy released and absorbed per gram of tissue in 1 s is 3.7 × 104 Ēβ MeV, where Ēβ is the average β-particle energy per disintegration, in mega–electron volts. The average β-particle energy per disintegration for 90Y is 0.93 MeV. One rad is defined as 100 erg/g of tissue. It is equivalent to the absorption of 6.24 × 107 MeV/g: Eq. 1where dis = disintegration.
Eq. 1where dis = disintegration.
The average half-life is used to determine the total dose received during treatment and is equal to the half-life multiplied by 1.44. The half-life for 90Y is 2.66 d. Therefore, the total dose for complete decay of 90Y is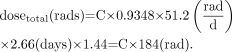 Eq. 2
Eq. 2
The administered 90Y-microsphere activity is distributed in tumor and normal liver compartments. The distribution profile is determined by the relative vascularity and volume of these 2 compartments and is expressed as the tumor-to-liver ratio (TLR). When lung shunting due to intrahepatic peritumoral arteriovenous communications occurs, a third compartment (lung) is encountered and is expressed as the lung shunt fraction (SF). The TLR and SF can be determined using 99mTc-macroaggregated albumin scans. Region-of-interest analysis of tumor and normal liver compartments on SPECT images is used to determine the TLR. The SF is calculated on planar images using the formula below: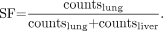 Eq. 3
Eq. 3
It is assumed that the administered activity is distributed evenly within the normal liver and tumor compartments. The tumor compartment, as expected, receives a higher concentration proportional to the TLR. Using the tumor and liver masses, the dose fraction accumulated in the normal liver (fractional liver uptake) is Eq. 4
Eq. 4
Activity to be administered for a desired liver dose can be calculated from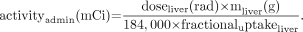 Eq. 5
Eq. 5
Dose delivered from a given administered activity is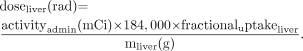 Eq. 6
Eq. 6
The fraction of the administered activity accumulated in the tumor (fractional tumor uptake) is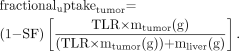 Eq. 7
Eq. 7
The dose to the tumor and the lungs can be determined using the following equations: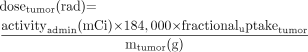 Eq. 8
Eq. 8 Eq. 9
Eq. 9
Mass is assumed to be equal to volume for tumor and liver tissues, because their densities are close to that of soft tissue (1.04 g/cm3). Therefore, for simplicity, “mass” can be replaced with “volume” in equations for liver and tumor dose determination. The density of lung, however, is approximately 0.30 g/cm3. Therefore, measured lung volumes on CT images need to be multiplied by this factor to obtain the mass. A lung mass of 1,000 g based on the anthropomorphic phantom design applied in MIRD modeling can be used if CT calculation is not available.
Concluding Notes
The MIRD schema and its applications for radiopharmaceuticals labeled with other pure-β-emitting radionuclides using bremsstrahlung imaging and quantitation have been reported (11,12). This technical review has focused on 90Y-microsphere dose determination based on 99mTc-macroaggregated albumin image quantitation. The 99mTc-macroaggregated albumin administration and acquisition protocols and processing techniques are important and are the subject of a separate technical report. We believe that the application of the MIRD schema to 90Y-microsphere treatment of primary and metastatic liver tumors offers better guidance than does empiric approaches. The development of more accurate absorbed dose estimates and the correlation of these estimates with biologic response will lead to a better understanding of the results of treatment and improve the clinical outcomes.
Footnotes
-
COPYRIGHT © 2006 by the Society of Nuclear Medicine, Inc.
References
- Received for publication November 30, 2005.
- Accepted for publication March 13, 2006.







