Abstract
Peptide receptor radionuclide therapy (PRRT) is an efficient treatment for gastroenteropancreatic neuroendocrine tumors (GEP NETs), with outstanding overall response rates and survival. However, little is known about the particular efficacy regarding bone metastasis (BM). Methods: We retrospectively analyzed a consecutive subgroup of 42 patients with BM of GEP NETs treated with PRRT (177Lu-octreotate, 4 intended cycles at 3 monthly intervals [10–14 wk]; mean activity per cycle, 8.1 GBq). Availability of restaging and outcome data was required for patient inclusion. Baseline characteristics, including age, tumor origin, performance score, Ki-67 index, tumor load, tumor uptake, plasma chromogranin A, and neuron-specific enolase, were analyzed regarding impact on tumor regression (modified M.D. Anderson criteria) and time to progression. Survival analyses were performed using Kaplan–Meier curves, log-rank test at a significance level of P less than 0.05, and Cox proportional hazards model for uni- and multivariate analyses. Results: Median follow-up was 32 mo. The observed response of BMs consisted of complete remission in 2 (4.8%), partial remission in 14 (33.3%), minor response in 5 (11.9%), stable disease in 16 (38.1%), and progressive disease in 5 (11.9%) patients. Median progression-free survival and overall survival (OS) were 35 mo (26–44, 95% confidence interval) and 51 mo (37–65, 95% confidence interval), respectively. Patients with responding BMs (complete remission, partial remission, or minor response) exhibited a trend toward better OS (median OS not reached after 53 mo) when compared to nonresponding patients (39 mo, P = 0.076). Only Ki-67 index (>10%) and chromogranin A level (>600 ng/mL) contributed to regression analysis. Conclusion: BM of GEP NETs is effectively controlled by PRRT, with long progression-free survival and OS. Poor patient condition and multifocality of BMs do not clearly affect treatment efficacy, possibly encouraging the use of PRRT in advanced bone metastatic disease. Larger studies are needed to assess predictors of treatment outcome in these patients.
- neuroendocrine tumors
- bone metastases
- peptide receptor radionuclide therapy
- 177Lu-DOTA octreotate
- oncology
The therapeutic management of gastroenteropancreatic neuroendocrine tumors (GEP NETs) is challenging in the metastatic, unresectable stage. Bone metastases (BMs) are present in 8%–19% of metastatic GEP NETs (1–6). They predominantly occur in patients with liver metastases (4,7,8), but bone-only disease has been observed. The clinical impact of BMs is significant; they cause pain and eventually decreased bone marrow function. However, apart from one study describing an impaired outcome in patients with nonhepatic distant metastases (2), there is little published data on the general prognostic impact of BM in NET (1,9).
Few studies have examined the influence of BMs on outcome. In the context of peptide receptor radionuclide therapy (PRRT), one study reports a negative impact of the presence of BMs on time to progression after 177Lu-octreotate (10). PRRT is an efficient systemic treatment for metastatic GEP NETs, producing outstanding overall response and survival (11). It is safe even when readministered during relapse of progressive disease (12,13). However, little is known about the particular efficacy of this treatment regarding BMs.
This retrospective study aims to assess the therapeutic effect of PRRT, namely with 177Lu-octreotate, on metastatic bone disease in a specific subgroup and identify potential risk factors for impaired outcome.
MATERIALS AND METHODS
Patients
Forty-two consecutive patients (mean age, 62 y; age range, 44–88 y; 26 men, 16 women) with BMs were retrospectively analyzed. All patients had well-differentiated GEP NETs, were treated with PRRT at our institution, and had their restaging completed. Twelve patients had pancreatic NET, and 30 patients had nonpancreatic GEP NETs, of which 4 had foregut, 9 midgut, 3 hindgut, and 12 other GEP NETs including an unknown primary. Eleven patients had metastatic bone pain. Apart from bone, metastatic sites included liver in 41 (97.6%), lymph nodes in 25 (59.5%), and other organs in 14 (33.3%) patients. Previous treatments included surgery (n = 22, 52.4%), biotherapy (n = 17, 40.5%), chemotherapy (n = 11, 26.2%), and locoregional treatment (n = 2, 4.8%).
Histopathology
Patients were classified according to the current TNM staging and grading system for NET (14,15). All tumors were well-differentiated endocrine carcinomas according to the histopathology with the presence of distant metastases (TNM stage IV). The histology and the Ki-67 proliferation index were determined out of resection specimens (n = 19; 45.2%) or biopsy material (n = 23; 54.8%) from liver metastases or the primary tumor. Immunohistochemical assessment of the Ki-67 index using the MIB1 antibody was expressed as percentage of stained tumor cells in 2,000 cells in areas in which the highest nuclear labeling was observed (14,15).
PRRT
Inclusion criteria for treatment with PRRT were histologically confirmed, unresectable, metastatic GEP NETs; sufficient tumor uptake (i.e., ≥liver uptake on baseline receptor imaging); a glomerular filtration rate of more than 30 mL/min/1.73 m2; a white blood cell count of 2 × 109/L or more; and platelets more than 70 × 109/L. PRRT was performed by administration of 8.1 ± 0.98 GBq of 177Lu-DOTA octreotate per treatment cycle, aimed at 4 courses at standard intervals of 3 mo (10–14 wk). The 177Lu (IDB Holland) had a specific activity in the range of 100–160 GBq/μmol at the time of administration. Peptide labeling (16,17) was performed to obtain apparent specific activity of about 54 GBq/μmol (ratio of activity to the total amount of peptide).
Response Assessment
Patients were restaged 3 mo after termination of PRRT. CT or MRI was performed according to the baseline imaging modality. Functional imaging was also performed consisting of somatostatin receptor scintigraphy (111In DTPA-octreotide [OctreoScan]; Covidien) or somatostatin receptor PET/CT (68Ga-DOTATOC) and bone scintigraphy. Follow-up imaging was usually performed at 6-mo intervals after the first restaging.
Overall tumor response in our center was reported according to the modified Southwest Oncology Group (SWOG) solid tumor response criteria (18,19), with minor response (MR) being defined as 25%–49% remission of the sum of products of perpendicular diameters of all measurable tumor lesions. Response of BMs, which classically reflect nonmeasurable disease in conventional imaging response criteria (response evaluation criteria in solid tumors [RECIST], SWOG), was determined according to functional M.D. Anderson criteria (20), modified for the purpose of assessment in NET. Complete remission (CR) was defined as complete resolution of all bone lesions in functional imaging; partial remission (PR) as complete disappearance of one or more bone lesions, together with substantial decrease in tracer uptake in the remaining lesions; MR as substantial decrease in tracer uptake in the bone lesions, without complete resolution of any lesion; stable disease as no significant change in functional imaging; and progressive disease (PD) as any new bone lesion.
Outcome and Statistical Analysis
The baseline characteristics of the study population were analyzed regarding the associated tumor response. For this purpose, the Fisher exact test was applied after dichotomizing for each factor and the resulting response: regression (CR, PR, or MR) versus nonregression (stable disease or PD) and progression (PD) versus nonprogression (CR, PR, MR, or stable disease).
Overall survival (OS) and progression-free survival were censored at the time of commencement of another significant treatment, such as chemotherapy or salvage PRRT, but not somatostatin medication. The underlying event for calculation of progression-free survival was documentation of progression of BMs. Survival analysis was performed using the Kaplan–Meier curve method. The log-rank test was carried out with a significance level of P less than 0.05. Univariate regression analysis by Cox proportional hazards model was performed for each baseline factor. Multivariate analysis (stepwise model by backward elimination) was performed with those variables contributing to the univariate model.
RESULTS
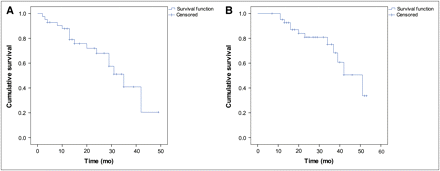
The median follow-up was 32 mo (95% confidence interval [CI], 29–35), and the median OS of the entire cohort (n = 42; Fig. 1) was 51 mo (95% CI, 37–65). Twelve patients (28.6%) had died by the end of the study. No treatment-related deaths were observed.
Kaplan–Meier curves for osseous progression-free survival (A) and OS (B) of entire study cohort (n = 42). Median progression-free survival was 35 mo (95% CI, 26–44) and median OS 51 mo (95% CI, 37–65).
The observed treatment response of BMs consisted of CR in 2 (4.8%), PR in 14 (33.3%), MR in 5 (11.9%), stable disease in 16 (38.1%), and PD in 5 (11.9%) patients. From the 11 patients with bone metastatic pain, 6 (55%) had complete and 5 (45%) partial resolution of symptoms. Median time to progression of BMs was 35 mo (95% CI, 26–44) from start of treatment; Figure 1 shows the respective Kaplan–Meier curve of the entire patient cohort.
Looking at the different baseline characteristics with regard to treatment response of BMs (Table 1), none of the examined variables were associated with an increased or decreased rate of regression (CR, PR, or MR). The association with the rate of treatment failure, that is, progression despite treatment, could not be determined because of the small PD group (n = 5), but again none of the baseline factors was significant.
Response of BMs to PRRT According to Various Baseline Characteristics
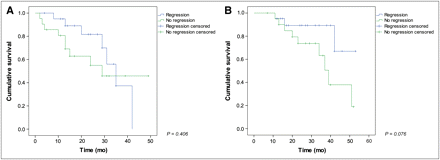
PRRT-induced regression of BMs (Fig. 2 illustrates an example case) in turn is associated with a trend toward improved outcome (Fig. 3). The regression group has not yet reached median OS after 53 mo of follow-up (with 71.4% of patients alive), whereas the median OS of the nonregression group is 39 mo (95% CI, 33–45; P = 0.076, log-rank test).
Regression of BMs illustrated by 68Ga-DOTATOC PET/CT before (A) and 3 mo after (B) PRRT in a patient with metastatic midgut carcinoid. Maximum-intensity-projection PET images (coronal views) are shown on left, fused and unfused CT images (selected lesion indicated by arrow) on right. This patient had functional remission of BMs accompanied by osteosclerotic changes, classified as PR. Patient remained in remission for 34 mo by end of study.
Patients showing regression of BMs after PRRT had similar progression-free survival (A) but trend toward prolonged OS (B) when compared with nonresponders (log-rank test, P = 0.076).
Risk factors for early progression of BMs after PRRT in the univariate analyses are a Ki-67 index greater than 10% and a chromogranin A level greater than 600 ng/mL (Table 2). Multivariate analysis leaves none of these 2 variables significant; however, Ki-67 index reaches borderline significance, with a hazard ratio (HR) of 3.4 (95% CI, 0.9–13.7; P = 0.083). The Kaplan–Meier curves, after dichotomizing for chromogranin A (Fig. 4) and Ki-67 index (Fig. 5), illustrate the prognostic value of these variables with regard to progression.
Uni- and Multivariate Analyses for Potential Factors Associated with Time to Progression of BMs
Progression-free survival (A) and OS (B) stratified by pretreatment chromogranin A plasma level (cutoff, 600 ng/mL).
Progression-free survival (A) and OS (B) stratified by tumor proliferation index (cutoff, 10%).
DISCUSSION
This retrospective study shows that BM of well-differentiated GEP NETs treated with PRRT can be controlled effectively over a long period. We found a median time to progression of approximately 3 y (35 mo), with a median OS in these advanced metastatic patients of 51 mo. Our findings support the few retrospective data existing on this GEP NET subgroup: in the largest study on PRRT in GEP NETs (21), Kwekkeboom et al. reported outcome results for 310 patients treated with 177Lu-octreotate, of which 68 had BMs. Median OS in this specific subgroup was reported to be 37 mo from start of treatment; further information, such as response or time to progression of BMs, is not available.
In our study, regression of BMs after PRRT as assessed by functional imaging is common (50% of all cases) and associated with a trend toward improved outcome. Patients in whom osseous tumor regression was achieved did not reach median OS after 53 mo, whereas median OS in the remaining patient group was 39 mo (3-y OS, 89.3% vs. 63.2%, respectively). The insignificant P (0.076) could be explained by an insufficient follow-up period in this particular setting with few events. However, any observation of improved survival in responding patients has to be viewed with particular caution because of the retrospective nature of the study.
BMs can cause pain, with a significant impact on quality of life. Few data are available for their symptomatic relevance in NET (9); in our cohort, 11 (26%) patients had metastatic bone pain. In these patients, treatment with PRRT led to complete (55%) or partial (45%) resolution of symptoms. The onset of pain alleviation was typically noted within a few weeks after the first treatment cycle, and the duration paralleled time to progression. This palliative potential is notable and relevant in the management of bone metastatic NET.
Analogous to the fact that well-responding GEP NETs exhibit a shorter time to progression (10,22), our retrospective study shows that osseous responders tend to catch up with nonresponders regarding progression-free survival after a certain interval (Fig. 3A). Although pancreatic NET are known to respond better to PRRT than GEP NETs of other origin (carcinoid tumors) according to morphologic (extraosseous) response criteria such as those of the World Health Organization, RECIST, or SWOG (10,22), we could not find a corresponding difference in response of BM. The regression rate was similar in pancreatic (58.3%) and nonpancreatic (46.6%) GEP NETs (P = 0.734). Also, progression-free survival of pancreatic NET was not significantly different from that of nonpancreatic NET, in our bone metastatic study cohort (29 vs. 35 mo, respectively). Although caution is required when interpreting retrospective results of a limited cohort (n = 42), this comparable outcome of BMs in pancreatic and nonpancreatic GEP tumors is noteworthy and may help to optimize patient management and scheduling of follow-up.
The only baseline variables contributing to progression-free survival in the univariate Cox proportional hazards model were the tumor marker level (chromogranin A > 600 ng/mL; HR, 3.2 [95% CI, 1.1–9.5]; P = 0.039) and the proliferation rate (Ki-67 index > 10%; HR, 5.2 [95% CI, 1.5–18.0]; P = 0.01). However, none of these proved significant on multivariate analysis, probably because of the small sample size. The chromogranin A level, which to some extent reflects overall tumor burden, has been found in some studies to be a negative predictor of outcome (4,23). Our finding confirms that a high chromogranin A level is a negative predictor for the particular setting of PRRT in the presence of BMs. However, multifocality of BMs, more specifically reflecting osseous tumor load than chromogranin A levels, was not a negative predictive factor of outcome after PRRT. This retrospective factor analysis should be viewed with caution in light of the relatively small number of the study population. This is also illustrated by the fact that the known predictive factor of tumor uptake (11,21), which can be seen as a surrogate for tumor absorbed dose, failed to show a significant impact on tumor regression in our analysis (P = 0.12).
The proliferation index has also been shown to affect outcome in the literature (6,24), which is not unexpected considering its biologic meaning. Our data confirm the propensity toward earlier progression with increased Ki-67 indices in our cohort of radiopeptide-treated BMs, irrespective of the initial treatment response recorded. To the best of our knowledge, there are no data available for comparison because no studies have evaluated the response and progression of BM of NET in the setting of any treatment modality. The parameter Ki-67 index is limited; it is rarely determined on BMs and may yield different results depending on the site and time point at which it is assessed.
Interestingly, the number of metastatic bone lesions that reflect osseous tumor burden as well as the Karnofsky performance score did not affect osseous tumor regression induced by PRRT (Table 1). Also, these 2 factors did not affect progression-free survival (Table 2). This finding is encouraging, because it might indicate that patients with advanced BMs and a reduced performance score may respond and benefit from PRRT in a way similar to patients with only a few (<10) bone lesions. Although comparable investigations on BM in the literature are lacking, analogous analyses for liver metastases have linked high tumor load and low performance status to poor outcome (21,25,26).
The main limitation of the study is the population size regarding statistical analyses of smaller subgroups. Particularly, the group of patients progressing despite PRRT (PD group; n = 5) was too small to permit valid analyses of this factor. Also, multivariate analysis should be viewed with caution in this respect. Strong predictors for negative treatment outcome after PRRT would be expected to become clear despite these caveats, even in this limited study cohort. However, as mentioned earlier in the discussion, one important predictive factor of treatment response, the grade of tumor uptake, failed to show significance for response in this analysis. The presented population, a group of 42 patients with BMs undergoing identical treatment, is an important and valuable study cohort for analyses in this rare entity. We recognize the additional limitation of the retrospective study design and its negative impact on the strength of our conclusions.
CONCLUSION
Our study shows that BM of well-differentiated gastroenteropancreatic NET are effectively controlled by PRRT, leading to long progression-free and overall survival as well as alleviation of pain if present. Treatment efficacy—that is, response and long-term inhibition of progression—is not clearly affected by multifocality of bone lesions or reduced patient condition. This may encourage the use of PRRT in advanced bone metastatic disease; however, larger studies are needed to confirm and expand these initial findings.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Professors Eric Krenning and Dik Kwekkeboom (Erasmus Medical Center, Rotterdam, Netherlands) for sharing their invaluable experience in the receptor-targeting field and making somatostatin receptor–mediated treatment at all possible in Bonn. Also, we thank the personnel of the Nuclear Medicine Department and especially the nursing staff of the therapy ward and Ulrike Kuhn-Seifer. No potential conflict of interest relevant to this article was reported.
- © 2011 by Society of Nuclear Medicine
REFERENCES
- Received for publication March 13, 2011.
- Accepted for publication May 16, 2011.