Abstract
123I-Metaiodobenzylguanidine (MIBG) accumulations that do not correspond to any tumor are observed occasionally on the medial aspect of the upper back or shoulder of children. The true nature of such accumulations is unknown, and we hypothesized that they represent interscapular brown adipose tissue (IBAT) visualized by scintigraphy. Methods: Wistar rats (7 wk old) received MIBG labeled with 123I or 125I. Autoradiography was performed, and concentrations of the tracer in the interscapular subcutaneous tissue were identified histopathologically. The effects of 6-hydroxydopamine, reserpine, and β 3-adrenergic receptor agonist (CL316,243) on the accumulation were investigated to elucidate the mechanism of uptake into BAT. Results: Autoradiography showed well-defined distinct accumulation in the subcutaneous tissue on the upper back, and hematoxylin-eosin and anti-uncoupling protein 1 antibody staining confirmed that it was BAT. The percentage injected dose per gram in BAT was as high as that in the heart and was quite different from the concentration in white adipose tissue. Preadministration of 6-hydroxydopamine or reserpine resulted in lower MIBG concentrations in BAT. Activation of the β3-adrenergic receptor accelerated the washout of MIBG in BAT and caused an increase in concentration in white adipose tissue. Conclusion: MIBG accumulates in the adrenergic nervous system in BAT, and IBAT is distinguished from the surrounding white adipose tissue. To our knowledge, BAT has not been visualized previously. We showed that MIBG scintigraphy might be suitable for the investigation of BAT and treatment of human obesity.
Metaiodobenzylguanidine (MIBG) is a derivative of guanethidine and acts as an analog of norepinephrine (1). MIBG labeled with 123I or 131I is widely used as an index of the integrity and function of the adrenergic nervous system, especially in the heart (2). It shares the same cellular transport system with norepinephrine, and MIBG acts like norepinephrine and accumulates in neuroendocrine tumors—that is, in pheochromocytoma, neuroblastoma, paraganglioma, and so forth (3–5).
In our experience with 123I-MIBG scintigraphy of neuroendocrine tumors, we sometimes noticed symmetric accumulation in the shoulder region of children that corresponded to nontumorous tissue. Such accumulation was seen in 27 of 260 studies; all of these positive studies were performed in the winter season (November to March in Japan) (Fig. 1), and it was seldom seen in adults. Moreover, the accumulation varied with examination, even in the same individual. Some authors have made the same observation (6–8). Bonnin et al. (6) stated that physiologic accumulation is sometimes seen in the upper thorax. Lumbroso et al. (7) reported that bilateral symmetric supraclavicular uptake, which was seen more clearly on posterior views and disappeared in a subsequent study, corresponded to pleural apex uptake. Elgazzar et al. (8) reported the same accumulation in 2 of 31 adult patients, and they attributed this accumulation to uptake by muscles. However, none of these authors had any evidence of the nature of the accumulation. In view of the site of the accumulation and the significant difference in its incidence between children and adults, brown adipose tissue (BAT) came mind as the true site of the accumulation.
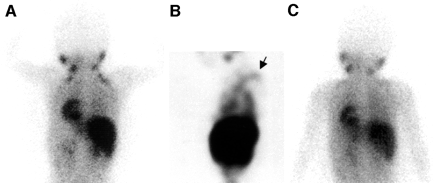
A 4-y-old boy treated for neuroblastoma. 123I-MIBG examinations were performed in winter (February). Posterior image (A) and coronal slice of SPECT image of chest (B) 6 h after injection of MIBG show hot spots on shoulder (arrow). Subsequent CT, physical examination, and tumor marker studies failed to detect any evidence of tumor at same site. (C) Next MIBG study performed in summer (August) did not show accumulation.
BAT is one organ that exists near large vessels, in the deep cervical, perirenal, and paraaortic regions, and in the interscapular site (9), where it is readily detectable as a thin kite-shaped structure (10). It was originally named for its color, which is attributable to its high vascularity and cytochrome content, reflecting high mitochondrial density. It was first identified as the hibernating gland of animals in the cervical region of embryos (11). The most important function of BAT is thought to be thermogenesis (9–12). At birth, many mammals, including humans, come out of the warm uterus and are exposed to a cold environment; the BAT of neonates is activated in response to cold exposure and produces heat to maintain the body temperature. Although its function decreases with aging in humans, some species of animals (rats and mice) and even adult humans, especially outdoor workers in northern Finland, retain active BAT, which plays an important role in cold-induced and diet-induced thermogenesis (9,10, 13). BAT is rich in sympathetic nerves, and the activation of BAT is mediated by the sympathetic nervous system (14–16). It is now established that 3 isoforms of β -adrenergic receptors are present in BAT; among them, the β3- adrenergic receptor plays the most important role in thermogenesis in BAT (16). The fact that BAT has an abundant sympathetic nervous supply and that human infants and children have more active BAT than adults seemed to support our hypothesis that MIBG accumulates in BAT.
The aim of this study was to investigate whether MIBG accumulates in the interscapular BAT (IBAT) of rats and, if it does accumulate, to elucidate the mechanism.
MATERIALS AND METHODS
Seven-week-old female Wistar rats (weight, 132.3–158.2 g; mean ± SD, 140 ± 4.5 g) were prepared for this study. Rats were the one of the most appropriate animals to use to determine whether MIBG accumulates in BAT because rats have a relative abundance of BAT. Neonates would be the most suitable subjects for experiments but, because they were not available, we used the youngest rats available for the experiments (i.e., 7 wk old). All rats were given KI (5 mg/d) through an oral tube for 3 d before administration of MIBG to avoid concentration of the tracer in the thyroid glands. The rats were anesthetized with an intraperitoneal injection of pentobarbital (35 mg/kg) before injection of the tracer. 123I-MIBG (27.8–35.2 GBq/mg) and 125I-MIBG (7.4 GBq/mg) (Daiichi Radioisotope Laboratories, Ltd., Chiba, Japan) were prepared. 125I-MIBG was used for studies requiring longer-lived radionuclides. To avoid the influence of environmental temperature on the activity of BAT, each entire experiment, except after the killing the animals, was performed at room temperature (20°C).
Autoradiography and Histologic Assessment
Three rats were injected with 925 MBq 125I-MIBG into the femoral vein; 30 min after the injection, the animals were killed by ether inhalation. The whole body was frozen in hexane cooled with dry ice, the extremities and tail were cut away, and the torso was completely embedded in 3% carboxylmethyl cellulose. The frozen body was cut into 50-μm-thick sagittal sections at the midline with a microtome (LKB2250 PMV Cryo-Microtome; LKB, Stockholm, Sweden); after mounting the sections on a vinyl sheet, they were dried in air. To visualize 125I-MIBG, an imaging plate (BAS1800; Fuji Photo Film, Tokyo, Japan) was exposed to the sections for 3 d.
After determination that the accumulation was in the subcutaneous tissue on the upper back, it was excised from another rat, and 5-μm-thick consecutive slices were cut and stained with hematoxylin-eosin. Because mitochondrial uncoupling protein 1 (UCP1) has usually been found only in BAT (16), the other 5-μm-thick tissue slices were stained with anti-UCP1 antibody to identify the accumulation as being in BAT.
Effect of 6-Hydroxydopamine and Reserpine
6-Hydroxydopamine (6-OH-DA) and reserpine were used in the experimental groups to estimate the extent to which sympathetic nervous activity contributes to the accumulation and how much MIBG is stored in the neurosecretory granules. Twenty-seven rats were divided into 3 groups: a 6-OH-DA group, a reserpine group, and a control group. Adrenergic neuron function was impaired in the 6-OH-DA group by the intraperitoneal injection of 6-OH-DA (100 mg/kg) 5 d before MIBG administration. To investigate whether the MIBG is stored in intracellular neuroendocrine vesicles, the system of uptake into the storage vesicles was selectively blocked by intraperitoneal administration of reserpine (4 mg/kg) 4 h before MIBG administration. We chose these doses and the duration of 6-OH-DA and reserpine on the basis of reports describing the sympathetic nerve function of the heart with MIBG (17,18).
Each rat was injected with 185 MBq 125I-MIBG into the femoral vein. At 5, 30, and 60 min after the injection of the tracer, 3 rats in each group were killed by ether inhalation, and the IBAT, heart, spleen, a section of white adipose tissue (WAT), and liver were excised. After weighing the tissues, their radioactivity was determined with an autogamma counter (MINAXI γ-AUTO-GAMMA 5000 series γ-counter; Packard Japan, Tokyo, Japan). Tissue concentrations are expressed as the percentage injected dose per gram (%ID/g) corrected for decay to normalize the differences in organs and individuals.
Effect of Specific β3-Adrenergic Receptor Agonist
CL316,243, disodium-(R,R)-5-[2-[2,3-(3-chlorphenyl)-2-hydroxyethyl-amino]propyl] -1,3-benzodioxole-2,2-dicarboxylate, is a β3-adrenergic receptor agonist that selectively stimulates the β3-adrenoreceptor (β1:β 2:β3 = 0:1:100,000) (19). Because BAT is rich in β3-adrenoreceptors, but other organs, including the heart, contain very few β3-adrenoreceptors, CL316,243 is used for selective activation of BAT (19,20). CL316,243 was provided by American Cyanamid Co. (Pearl River, NY).
To assess the relationship between MIBG accumulation and the adrenergic nervous system activity in BAT, 9 animals were injected intraperitoneally with 0.1 mg/kg of CL316,243 dissolved in physiologic saline at a concentration of 0.05 mg/mL, whereas 9 rats received saline as a control group. One hour after administration of CL316,243 or saline, each rat received an injection of 1,850 kBq 123I-MIBG into the femoral vein. Three rats in each group were killed, at 5, 30, and 60 min after tracer injection. Samples of BAT, heart, and WAT were collected, and the tissue %ID/g was calculated in the same way as described for the experiments using 6-OH-DA and reserpine. In this experiment, the radioactivity was determined with the other autogamma counter (WIZARD 3″ 1480 Automatic γ-counter; Wallac Berthold, Tokyo, Japan).
Statistical Analysis
Statistical analyses were performed using the nonparametric Wilcoxon test to evaluate the significance of differences in the values between the control and experimented animals. Acceptable probability for a significant difference between means was P < 0.05.
RESULTS
MIBG Distribution and Histopathologic Study
Figure 2 shows a representative whole-body midsagittal section and the corresponding autoradiogram 30 min after injection. Some isolated accumulations were evident in the subcutaneous tissue of the upper back. The medial incision using the posterior neck approach revealed a brown well-defined kite-shaped structure surrounded by subcutaneous WAT, and it corresponded to the site where MIBG had accumulated. Figure 3 shows the morphologic characteristics of this structure. Microscopically, the tissue volume was filled with polygonal multilocular cells (Fig. 3A, hematoxylin-eosin stain), which were positive for anti-UCP1 antibody (Fig. 3B). Thus, the tissue containing the concentration of MIBG was identified as BAT.
Midsagittal section of whole body (A) and 125I-MIBG autoradiogram (B). Some well-defined intense accumulations in subcutaneous soft tissue on upper back are evident (arrows).
Hematoxylin-eosin stain (×80) (A) and anti-UCP1 antibody stain (×80) with additional hematoxylin stain (B) of sections of tissues in which MIBG was concentrated. Tissue was composed of multilocular cells that were positive for UCP1 antibody.
Pharmacologic Inhibition of Adrenergic Nervous System and Blockage of Uptake into Neuroendocrine Storage Vesicles
The results in the control and pretreated rats are summarized in Table 1. The %ID/g data are presented as mean ± SD.
Summary of Results of Control Rats and Rats Pretreated with 6-OH-DA and Reserpine
Figure 4 shows the time course of %ID/g in the organs of the control group. The tissue concentrations in BAT were significantly higher than those in the liver, spleen, and WAT. Although they showed faster washout than the accumulation in the heart, the concentrations in the early phase were as high as that in the heart.
Time course of %ID/g of 125I-MIBG in organs of control group. Measurements of accumulations are shown as mean values. %ID/g in BAT was much higher than that of liver, spleen, and WAT; at 5 and 30 min, values were same as those of heart. They showed faster washout than that of heart samples.
The 125I-MIBG concentrations in BAT were reduced in the 6-OH-DA and reserpine groups (Fig. 5). Five minutes after injection a decrease in uptake was seen only in the reserpine group, but it was not statistically significant. However, at 30 and 60 min, statistically significant decreases in concentration were observed in both groups. As for the other organs, the concentrations in the heart and spleen were also decreased by pretreatment with both drugs, but the concentrations in the WAT and liver were unchanged. Figure 6 compares the results for BAT in the 3 groups.
Time course of %ID/g of 125I-MIBG in organs of groups pretreated with 6-OH-DA) (A) and reserpine (B). Measurements of accumulations are shown as mean values. 125I-MIBG concentrations in BAT were reduced in both groups.
Effect of 6-OH-DA and reserpine on concentration of 125I-MIBG in BAT. Results for accumulation in BAT are presented as %ID/g. Mean %ID/g values + SDs are shown. *P < 0.05, **P < 0.01, ***P < 0.005 compared with controls. At 30 and 60 min after injection, %ID/g of rats treated with each drug showed significant decrease. At 5 min, reserpine-treated rats had lower accumulation (not significant).
Pharmacologic Stimulation by β3-Adrenergic Receptor Agonist
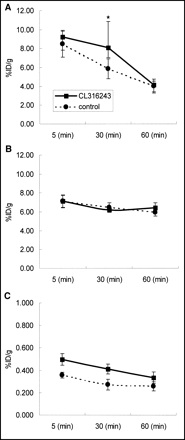
Macroscopically, the BAT in the treated group was browner than that of the control group. Figure 7 shows the time course of the %ID/g values in each organ. In BAT, the % ID/g in the experimental group (pretreated with CL316,243) was higher than that in the control group at both 5 and 30 min. The difference between the 2 groups was not statistically significant at 5 min but was significant at 30 min. At 60 min, the %ID/g in the experimental group had decreased more steeply than that of the control group. The %ID/g of the heart showed no response to the treatment. On the other hand, the %ID/g of WAT in the experimental group showed a significant increase at each time and, as in BAT, the concentration decreased rapidly compared with that of the control group.
Effect of CL316,243 on concentration of 123I-MIBG in BAT (A), heart (B), and WAT (C). Mean % ID/g values ± SDs are shown. *P < 0.05 compared with controls. %ID/g in BAT of CL314,243-treated group showed significant increase at 30 min, and rapid washout was seen at 60 min. In heart, no differences were found between control group and CL316,243-treated group. In WAT, concentrations in CL314,243-treated group were significantly higher than those of control group.
DISCUSSION
We investigated the concentration of MIBG in the IBAT of rats. Autoradiography revealed isolated accumulations at the expected sites, and the morphologic characteristics confirmed uptake by the BAT. The concentration of MIBG in BAT was much higher than that in the WAT, and 30 min after injection the %ID/g was as high as that in the heart.
It has been suggested that MIBG shares the same uptake, storage, and release mechanism as norepinephrine in the adrenergic nerve terminals, and MIBG is thought to behave in the same way as norepinephrine (1,21). A reserpine-blocking effect on MIBG accumulation has been reported in the heart and spleen (17), and MIBG uptake in the heart and salivary glands was found to be decreased by injury to adrenergic neurons (18,22). In our studies, both 6-OH-DA and reserpine decreased the accumulation in BAT, but neither drug affected its accumulations in the WAT and liver. The fact that treatment with 6-OH-DA and reserpine reduced the %ID/g of BAT indicated a specific contribution of sympathetic innervation to the accumulation of MIBG in BAT.
Whereas the reserpine-treated group showed a rapid decrease (at 5 min) in accumulation in BAT, the inhibition occurred later in the 6-OH-DA-treated groups. Physiologically, the thermogenic activity of BAT requires a very high perfusion rate through its vascular system, both to supply oxygen and substrate to the mitochondria and to export the heat produced (12). Therefore, the abundant blood flow might play a part in the role in accumulation of the tracer in BAT, regardless of the sympathetic nerve system. On the other hand, the finding that reserpine decreased the early accumulation implies a storage mechanism in the neuroendocrine vesicles of adrenergic neurons in BAT.
The selective stimulation of the β3-adrenergic receptor with CL316,243 caused a mild increase in the accumulation of MIBG in the BAT in the early phase, and rapid washout, and the accumulation decreased to as low as that in the control groups at 60 min, whereas accumulation in the heart was unaffected completely. When estimating the sympathetic nervous system function in the heart, it has been confirmed that hearts with impaired autonomic function show a decreased accumulation of 123I-MIBG (23) and that the hearts with compensatory activated sympathetic function in cardiac failure show an increased washout rate (24). It seems reasonable to assume that the same observation would apply to BAT.
The concentration in WAT was significantly higher in the CL316,243-treated group than that of the control group, although the level of accumulation was much lower than that in BAT. β3-Adrenergic receptor agonists are capable of inducing mitochondrial UCP1 and chronic treatment with CL316,243 induces UCP expression not only in BAT but also in various fat pads usually considered to be WAT, even in skeletal muscle (20). The authors have reported that UCP1-expressing fat pads contain numerous multilocular cells that are indistinguishable from typical BAT. Our results after a single administration of CL316,243 may reflect this phenomenon.
Recently, BAT has become more interesting in terms of its relationship to obesity (25–27) than to thermogenesis in the infantile period. A subgroup of obese persons has low BAT activity (25), and a mutation of the β3-adrenergic receptor gene has been reported in obese persons and patients with non- insulin-dependent diabetes mellitus (26). Ectopic expression of UCP induced by chronic administration of β3-adrenergic receptor agonists in WAT or muscle is an expected mechanism of a drug having an antiobesity effect (27).
Our study revealed accumulation of MIBG in the BAT of rats. Further studies are necessary to determine its significance in human imaging, but scintigraphy might be useful for investigating BAT and obesity.
CONCLUSION
Our experiments on rats revealed that BAT actually does concentrate MIBG. However, this finding cannot be considered solid evidence that the sites of MIBG accumulation on the shoulder and posterior neck of human infants and children correspond to BAT. Although several studies have been conducted on physiologic function and genetic relationships, no methods of visualizing BAT, other than incision of organ samples, have been available. To our knowledge, this is the first report on the visualization of BAT. The mechanism of the accumulation of MIBG in BAT is related to its sympathetic nerve function.
Footnotes
Received Dec. 3, 2001; revision accepted Apr. 26, 2002.
For correspondence or reprints contact: Chio Okuyama, MD, Department of Radiology, Kyoto Prefectural University of Medicine, 465 Kajii-cho Kawaramachi Hirokoji Kamigyo-ku, Kyoto 602-8566, Japan.
E-mail address: chio{at}koto.kpu-m.ac.jp