Abstract
1-11C-Octanoate is a potential tracer for studying astroglial function in PET. To evaluate the usefulness of 1-11C-octanoate for studying ischemic stroke, we investigated the brain distribution of 1-14C-octanoate and compared it with N-isopropyl-p-123I-iodoamphetamine (123I-IMP) distribution (cerebral blood flow), 123I-iomazenil (123I-IMZ) distribution (neuronal viability based on 123I-IMZ binding to benzodiazepine receptors), and hematoxylin-eosin stain (morphologic changes) in a rat model of focal cerebral ischemia. Methods: The right middle cerebral artery of each rat was occluded intraluminally. The brain distribution of 1-14C-octanoate and 123I-IMP (or 123I-IMZ) was determined 4 and 24 h after the insult using a dual-tracer autoradiographic technique (n = 4–7 in each group). Coronal brain sections adjacent to those used for autoradiography were stained with hematoxylin and eosin. Regions of interest (ROIs) were determined for 3 coronal slices, and asymmetry indices (AIs, lesion/normal hemisphere) of the tracer uptake were calculated. ROIs on the hemisphere with the lesion were classified into 4 groups: In region A, widespread necrotic cells were observed; in region B, necrotic cells were occasionally observed; in region C1, no morphologic changes were observed and the AIs for 123I-IMP (or 123I-IMZ) were ≤0.8; and in region C2, no morphologic changes were observed and the AIs for 123I-IMP (or 123I-IMZ) were >0.8. Results: 1-14C-Octanoate uptake decreased in the regions where morphologic changes were observed (regions A and B) but was relatively preserved in the surrounding region without morphologic changes despite reduced 123I-IMP and 123I-IMZ uptake (region C1). In the region without morphologic changes (region C1), AIs for 1-14C-octanoate were significantly higher than those for 123I-IMP (4 h, 0.73 ± 0.23 for 1-14C-octanoate and 0.37 ± 0.20 for 123I-IMP, P < 0.0001; 24 h, 0.84 ± 0.11 for 1-14C-octanoate and 0.44 ± 0.15 for 123I-IMP, P < 0.0001) and those for 123I-IMZ (4 h, 0.83 ± 0.19 for 1-14C-octanoate and 0.57 ± 0.13 for 123I-IMZ, P < 0.0001; 24 h, 0.91 ± 0.13 for 1-14C-octanoate and 0.73 ± 0.06 for 123I-IMZ, P < 0.0001). Conclusion: 1-14C-Octanoate uptake was relatively preserved in the regions without morphologic changes despite reduced 123I-IMP and 123I-IMZ uptake. 1-11C-Octanoate may provide further functional information on the pathophysiology of ischemic stroke, reflecting astroglial function based on fatty acid metabolism.
Although ischemic stroke is one of the most common neuronal disorders, the routine use of PET for the clinical assessment of pathophysiologic changes in the disease has been limited exclusively to the determination of cerebral blood flow (CBF) and oxygen and glucose metabolism (1), except for several laboratories. This restriction is due primarily to the lack of radiopharmaceuticals suitable for imaging the pathophysiology of ischemic stroke in clinical diagnosis of the disease. Recently, increasing interest has been focused on interactions between glial cells (particularly astrocytes) and neurons (2–5). It is now a matter of great importance to elucidate astroglial function, including metabolic function, in the pathophysiology of cerebral ischemia (6,7). The use of radiopharmaceuticals, combined with PET, for studying such astroglial function should provide useful information on the pathophysiology of the disease. To the best of our knowledge, however, there have been no reports concerning radiopharmaceuticals for imaging astroglial function, except for a preliminary report of Muir et al. (8). They proposed positron-labeled acetate and fluoroacetate as markers of glial cells, based on their basic studies with tritium-labeled compounds. However, the application of these compounds as in vivo imaging agents may be hampered by their low permeability of the blood-brain barrier (9).
Octanoate, an 8-carbon monocarboxylate, is converted to glutamine in astrocytes through the tricarboxylic acid cycle after β-oxidation (10–15), a process similar to the metabolism of acetate (8,16). In addition, octanoate is taken up in the brain more readily than are other mono-, di-, or tricarboxylic saturated fatty acids (9,17,18). Thus, a positron-labeled octanoate may be applicable as a PET tracer for studying cerebral astroglial function based on fatty acid metabolism and may be superior to positron-labeled acetate and fluoroacetate. In this regard, we previously evaluated the pharmacokinetic properties of radiolabeled octanoate and its derivatives in vitro (19,20) and in vivo (15,21–23) and demonstrated the potential of 1-11C-octanoate as a PET tracer for studying such astroglial function. We also preliminarily assessed brain uptake of 1-11C-octanoate in rat and canine models of focal cerebral ischemia (24,25).
In the present study, we investigated the brain distribution of 1-14C-octanoate and compared it with N-isopropyl-p-123I-iodoamphetamine (123I-IMP) distribution (CBF), 123I-iomazenil (123I-IMZ) distribution (neuronal viability based on 123I-IMZ binding to benzodiazepine receptors), and the results of hematoxylin-eosin (HE) staining (morphologic changes) in a rat model of focal cerebral ischemia, to further evaluate the usefulness of 1-11C-octanoate for studying ischemic stroke.
MATERIALS AND METHODS
1-14C-Octanoate in ethanol (radiochemical purity, >99%; specific activity, 1.96 GBq/mmol) was purchased from American Radiolabeled Chemicals Inc. The ethanol solution of 1-14C-octanoate was evaporated under a nitrogen stream, and the residue was dissolved in physiologic saline (3.0 MBq/mL). 123I-IMP and 123I-IMZ were synthesized according to the methods reported previously (26,27). All other reagents used were of analytic grade.
Animal Preparation
The experimental protocol was fully approved by the Laboratory Animal Care and Use Committee of Hokkaido University. Male Sprague-Dawley rats weighing 300–350 g were used. The rats were allowed free access to water and laboratory chow.
The rats were anesthetized intraperitoneally using chloral hydrate (400 mg/kg body weight). The ostium of the right middle cerebral artery (MCA) in each rat was occluded intraluminally following a method described in detail previously (28–30). The rats were allowed to recover from anesthesia, and any induced neurologic deficits were confirmed. Rats not showing any neurologic deficits were excluded from the study.
Autoradiographic Studies
The brain distribution of 1-14C-octanoate was determined and compared with CBF at 4 (n = 4) and 24 (n = 4) h after the insult using a dual-tracer autoradiographic technique with 1-14C-octanoate and 123I-IMP. A dose of 1-14C-octanoate (3.0 MBq/kg body weight) was injected first through the femoral vein. 123I-IMP (111 MBq/kg body weight) was then injected 5 min later through the contralateral femoral vein. These tracers were injected while the rats were under light ether anesthesia. Under ether anesthesia, the rats were sacrificed by decapitation 10 min after injection of 1-14C-octanoate, which occurred 4 and 24 h after the insult. The brain distribution of 1-14C-octanoate was also compared with 123I-IMZ distribution, a marker of neuronal viability based on benzodiazepine receptor function, 4 (n = 6) and 24 (n = 7) h after the insult. A dose of 123I-IMZ (111 MBq/kg body weight) was injected first through the femoral vein. Then, 50 min later, 1-14C-octanoate (1.5 MBq/kg body weight) was injected through the contralateral femoral vein. These tracers were injected while the rats were under light ether anesthesia. Under ether anesthesia, the rats were sacrificed by decapitation 10 min after injection of 1-14C-octanoate, which occurred 4 and 24 h after the insult.
The brains of the rats were removed and immersed in ice-cold saline. The brains were then sectioned at 2-mm thickness using a brain matrix (RBM-4000C; ASI Instruments) to obtain 8 coronal slices. The brain slices 4–6, 8–10, and 12–14 mm caudal from the frontal pole were incubated in a 1% solution of 2,3,5-triphenyl-tetrazolium chloride (TTC) at 37°C for vital staining. The brain slices 2–4, 6–8, and 10–12 mm caudal from the frontal pole were embedded in a medium (Tissue-Tek; Sakura Finetechnical Co., Ltd.), frozen in isopentane-dry ice, and cut into 20-μm sections with a cryostat (Bright Instrument Co., Ltd.).
The first autoradiographic exposure was performed for 3 h to detect the distribution of 123I-IMP or 123I-IMZ. The second exposure was initiated 10 d (18 half-lives of 123I) later and performed for 3 d to image the distribution of 1-14C-octanoate. It was preliminarily confirmed that the cross-contamination of 123I and 14C was less than 2%.
The coronal brain sections (20 μm) adjacent to those used for the autoradiographic studies were stained with HE.
Data Analysis
The autoradiographic images were analyzed using a computerized imaging analysis system (Bio-Imaging Analyzer BAS 5000; Fuji Photo Film). To quantitatively evaluate the distribution of 1-14C-octanoate and 123I-IMP, circular regions of interest (ROIs, 1.1 mm in diameter) were determined for 3 coronal brain slices as shown in Figure 1A. Because benzodiazepine receptor binding can reliably be assessed only in the cortex (31), only cortical regions were used for the comparative analysis of 1-14C-octanoate and 123I-IMZ. Asymmetry indices (AIs), defined as the ratios of values for an ROI in the right hemisphere to values for the contralateral homologous ROI and calculated after background subtraction, were used to exclude the effects associated with variations in levels of anesthesia. ROIs determined on the hemisphere with the lesion were classified visually in a masked manner into 3 groups based on the histologic findings of HE staining as follows (Table 1; Figs. 1B and 1C): In region A, widespread necrotic cells were observed; in region B, necrotic cells were occasionally observed; and in region C, no morphologic changes were observed. Cellular alterations such as pyknosis, eosinophilia, loss of hematoxylinophilia, scalloping, shrinkage, and swelling were considered necrotic, according to the criteria described by Garcia et al. (32). To characterize the 1-14C-octanoate distribution in morphologically normal regions with reduced 123I-IMP (or 123I-IMZ) uptake, ROIs assigned for region C were further divided into 2 groups based on AIs for 123I-IMP (or 123I-IMZ): In region C1IMP (region C1IMZ for 123I-IMZ), AIs for 123I-IMP (or 123I-IMZ) were ≤0.8; in region C2IMP (region C2IMZ for 123I-IMZ), AIs for 123I-IMP (or 123I-IMZ) were >0.8 (Table 1). An AI threshold value, 0.8, was chosen, considering the lesion detectability of the autoradiographic methods (33).
(A) Example of ROIs placed 2–4, 6–8, and 10–12 mm caudal from frontal pole on 3 coronal images. (B) Representative images of HE stain (×200). (C) Example of classified ROIs determined on images of HE stain. Black circle = region A; green circle = region B; blue circle = region C. (D) Approximate relationship of ROI classification with TTC stain. Black circle = region A; green circle = region B; blue circle = region C.
Classification of ROIs
The Bland-Altman plot (34), a graphical technique that helps assess agreement of 2 measurements, was used to compare AIs for 1-14C-octanoate with those for 123I-IMP or 123I-IMZ. A paired t test was used to assess the significance of differences in AIs between 123I-IMP (or 123I-IMZ) and 1-14C-octanoate. Statistical significance was determined using a 2-tailed value of P < 0.05/4 (i.e., 0.0125), considering a type I error rate for multiple comparisons.
RESULTS
Comparison with 123I-IMP Distribution (CBF)
Figure 2 shows the representative images of TTC staining and autoradiograms for 123I-IMP and 1-14C-octanoate. Both 123I-IMP uptake and 1-14C-octanoate uptake were markedly decreased in the infarct regions (TTC-unstained region) at 4 and 24 h after the insult. In contrast, 1-14C-octanoate uptake was relatively preserved in the periinfarct regions with reduced 123I-IMP uptake.
Representative images of TTC staining and autoradiograms for 123I-IMP and 1-14C-octanoate at 4 (A) and 24 (B) h after insult. 1-14C-Octanoate uptake decreased in infarct regions (TTC-unstained region), indicated by red arrows, but was relatively preserved in periinfarct regions with reduced 123I-IMP uptake, indicated by black arrows.
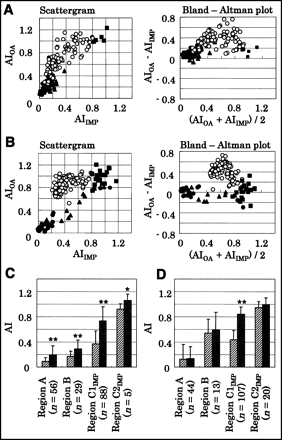
Scattergrams and Bland-Altman plots (34) of AIs for 123I-IMP versus AIs for 1-14C-octanoate are shown in Figures 3A and 3B. In the regions where morphologic changes were observed (regions A and B), AIs for both 123I-IMP and 1-14C-octanoate markedly decreased at 4 and 24 h after the insult. In the region without morphologic changes (region C), particularly in region C1IMP, 1-14C-octanoate uptake was relatively preserved, compared with 123I-IMP uptake.
(A and B) Scattergrams and Bland-Altman plots between AIs for 123I-IMP and AIs for 1-14C-octanoate 4 (A) and 24 (B) h after insult. • = region A; ▴ = region B; ○ = region C1IMP; ▪ = region C2IMP, AIIMP = AI for 123I-IMP; AIOA = AI for 1-14C-octanoate; regions A, B, C1IMP, and C2IMP are classified in Table 1. (C and D) AIs for 123I-IMP and 1-14C-octanoate in each region at 4 (C) and 24 (D) h after insult. Hatched bars = 123I-IMP; solid bars = 1-14C-octanoate. *P < 0.0125 vs. 123I-IMP. **P < 0.0001 vs. 123I-IMP.
Mean AIs for 123I-IMP and 1-14C-octanoate in each region are also summarized in Figures 3C and 3D. In region A, AIs were 0.09 ± 0.06 and 0.13 ± 0.24 for 123I-IMP and 0.20 ± 0.15 and 0.14 ± 0.19 for 1-14C-octanoate at 4 and 24 h, respectively, after the ischemic insult. The difference in AIs between 123I-IMP and 1-14C-octanoate was significant at 4 h (P < 0.0001). AIs in region B were 0.17 ± 0.08 and 0.54 ± 0.23 for 123I-IMP and 0.29 ± 0.14 and 0.59 ± 0.28 for 1-14C-octanoate at 4 and 24 h, respectively, after the insult. The difference in AIs between 123I-IMP and 1-14C-octanoate was significant at 4 h (P < 0.0001). In region C1IMP, AIs for 1-14C-octanoate were 0.73 ± 0.23 and 0.84 ± 0.11 at 4 and 24 h, respectively—significantly higher than those for 123I-IMP (0.37 ± 0.20 at 4 h, P < 0.0001, and 0.44 ± 0.15 at 24 h, P < 0.0001). AIs in region C2IMP were 0.92 ± 0.09 and 0.95 ± 0.09 for 123I-IMP and 1.06 ± 0.10 and 0.99 ± 0.12 for 1-14C-octanoate at 4 and 24 h, respectively, after the insult. The difference in AIs between 123I-IMP and 1-14C-octanoate was significant at 4 h (P < 0.0125).
Comparison with 123I-IMZ Distribution
The autoradiograms for 123I-IMZ and 1-14C-octanoate show that both 123I-IMZ uptake and 1-14C-octanoate uptake markedly decreased in the infarct regions (TTC-unstained region) at 4 and 24 h after the insult (Fig. 4). In contrast, 1-14C-octanoate uptake was relatively preserved in the periinfarct regions with reduced 123I-IMZ uptake.
Representative images of TTC staining and autoradiograms for 123I-IMZ and 1-14C-octanoate at 4 (A) and 24 (B) h after insult. 1-14C-Octanoate uptake decreased in infarct regions (TTC-unstained region), indicated by red arrows, but was relatively preserved in peri-infarct regions with reduced 123I-IMZ uptake, indicated by black arrows.
AIs for 1-14C-octanoate were markedly decreased at 4 and 24 h after the insult in the regions where morphologic changes were observed (regions A and B) (Figs. 5A and 5B). Relatively higher AIs for 123I-IMZ were occasionally observed in these regions. In the region without morphologic changes (region C), particularly in region C1IMZ, 1-14C-octanoate uptake was relatively preserved, compared with 123I-IMZ uptake.
(A and B) Scattergrams and Bland-Altman plots between AIs for 123I-IMZ and AIs for 1-14C-octanoate at 4 (A) and 24 (B) h after insult. • = region A; ▴ = region B; ○ = region C1IMZ; ▪ = region C2IMZ, AIIMZ = AI for 123I-IMZ; AIOA = AI for 1-14C-octanoate; regions A, B, C1IMZ, and C2IMZ are classified in Table 1. (C and D) AIs for 123I-IMZ and 1-14C-octanoate in each region at 4 (C) and 24 (D) h after insult. Hatched bars = 123I-IMZ; solid bars = 1-14C-octanoate. *P < 0.0125 vs. 123I-IMZ. **P < 0.0001 vs. 123I-IMZ.
In region A, AIs for 1-14C-octanoate were 0.14 ± 0.11 and 0.24 ± 0.14 at 4 and 24 h, respectively, after the ischemic insult—significantly less than those for 123I-IMZ (0.36 ± 0.23 at 4 h, P < 0.0001, and 0.43 ± 0.25 at 24 h, P < 0.0001) (Figs. 5C and 5D). AIs in region B were 0.52 ± 0.16 and 0.74 ± 0.17 for 123I-IMZ and 0.50 ± 0.17 and 0.71 ± 0.16 for 1-14C-octanoate at 4 and 24 h, respectively. The differences in AIs between 123I-IMZ and 1-14C-octanoate were not significant. In region C1IMZ, AIs for 1-14C-octanoate were 0.83 ± 0.19 and 0.91 ± 0.13 at 4 and 24 h, respectively—significantly higher than those for 123I-IMZ (0.57 ± 0.13 at 4 h, P < 0.0001, and 0.73 ± 0.06 at 24 h, P < 0.0001). AIs in region C2IMZ were 0.87 ± 0.05 and 0.90 ± 0.09 for 123I-IMZ and 1.05 ± 0.18 and 1.00 ± 0.09 for 1-14C-octanoate at 4 and 24 h, respectively, after the insult. These differences in AIs between 123I-IMZ and 1-14C-octanoate were also significant (P < 0.0125 for 4 h and P < 0.0001 for 24 h).
DISCUSSION
To evaluate the usefulness of 1-11C-octanoate for studying ischemic stroke, we investigated the brain distribution of 1-14C-octanoate and compared it with 123I-IMP distribution (CBF), 123I-IMZ distribution (neuronal viability based on 123I-IMZ binding to benzodiazepine receptors), and the results of HE staining (morphologic changes) in a rat model of focal cerebral ischemia. 1-14C-Octanoate uptake decreased in the regions where morphologic changes were observed (regions A and B) but was preserved in the region without morphologic changes despite reduced 123I-IMP and 123I-IMZ uptake (region C1). Thus, 1-14C-octanoate showed the characteristic brain distribution in a rat model of focal cerebral ischemia, indicating that octanoate labeled with 11C provides further functional information on the pathophysiology of ischemic stroke.
The present results show that an increased accumulation of 1-14C-octanoate relative to CBF takes place in ischemic but viable regions, indicating that some mechanism responsible for the increased trapping of 1-11C-octanoate-derived radioactivity is operating in these regions. These results are consistent with those of our previous study of a canine model of focal cerebral ischemia (25). Based on findings from previous studies on the brain uptake and metabolic aspects of octanoate (13,15), this trapping of radioactivity seems to be primarily attributable to 1-14C-octanoate metabolites, most likely labeled glutamate and glutamine, in astrocytes. The important contribution of astrocytes to brain uptake of octanoate has also been demonstrated (19,20). The relatively preserved 1-14C-octanoate uptake appears to reflect astroglial function based on octanoate metabolism.
123I-IMZ, a benzodiazepine partial-inverse agonist, has been developed to permit SPECT investigation of central benzodiazepine receptors and used for the detection of viable cortical neurons in ischemic stroke (33,35–37). Our results showed that uptake of 1-14C-octanoate and 123I-IMZ decreased in the region where necrotic cells were occasionally observed (region B), whereas AIs for 1-14C-octanoate were significantly higher than those for 123I-IMZ in the region without morphologic changes (region C). It is well known that glial cells are less sensitive to ischemic stress than are neurons (38–40). These findings also support the potential of radiolabeled octanoate for studying ischemic stroke as a marker of glial function.
The clinical use of PET for the assessment of pathophysiologic changes in ischemic stroke has been limited almost exclusively to the determination of CBF and of oxygen and glucose metabolism (1). 1-11C-Octanoate may provide us with a unique means of elucidating the pathophysiology of ischemic stroke. Particularly, 1-11C-octanoate, combined with 18F-FDG, may be useful for achieving better understanding of the contributions of glial cells to brain function and interactions between neurons and glial cells and may provide further diagnostic values for patients with ischemic stroke. It remains unclear, however, whether preserved 1-14C-octanoate uptake in the ischemic regions implies salvageable tissues or not. The precise mechanism underlying the trapping of octanoate, and the functions that are actually imaged by radiolabeled octanoate, also remain to be elucidated. Ischemic responses, such as astroglial activation, increased oxygen extraction rate, tissue acidosis, and changes in glutamate turnover, may influence the brain distribution of octanoate. Further studies, particularly those on the relationship between octanoate accumulation and the pathophysiology of cerebral ischemia, including oxygen and glucose metabolism and glial reactions, are required to clarify our understanding of this process.
1-11C-Octanoate is readily synthesized in reproducible high yields using a Grignard reaction of 11C-CO2 with heptylmagnesium bromide and a commercially available automated synthetic apparatus. The availability of this procedure is expected to favor the clinical application of 1-11C-octanoate. Conversely, clinical application of 1-11C-octanoate may be restricted by the intricacy of its metabolism, which renders analysis of its pharmacokinetics difficult. Developing kinetic models for the analysis of the pharmacokinetics of 1-11C-octanoate in the brain should ultimately prove to be of great benefit.
In the present study, the Bland-Altman plot (34) was used to assess the difference in AIs between 1-14C-octanoate and 123I-IMP (or 123I-IMZ). This graphical technique clearly showed that the AIs for 1-14C-octanoate were relatively higher than those for 123I-IMP and 123I-IMZ in the regions without morphologic changes (region C1), indicating that 1-14C-octanoate uptake was relatively preserved in the region (region C1), compared with 123I-IMP and 123I-IMZ uptake.
It is important to consider several methodologic aspects in the present study. In a clinical setting, several hours are required to sufficiently characterize benzodiazepine receptor distribution after 123I-IMZ injection. In this study, however, rats were sacrificed at 60 min after 123I-IMZ administration, according to the method reported by Toyama et al. (37). They indicated by a kinetic study that specific binding of 123I-IMZ can be evaluated at 60 min after injection in a rat model of cerebral ischemia. Specific distribution of 123I-IMZ appears to be achieved within a shorter time of 60 min in rats. Ex vivo uptake (binding) studies using 1-14C-octanoate and 123I-IMZ are helpful to confirm this point and to further characterize 1-14C-octanoate uptake in the brain. Such ex vivo studies, however, remain to be performed.
Although 123I-IMZ, a benzodiazepine partial-inverse agonist, has been used as a marker of neuronal viability, 123I-IMZ uptake decreased in the region C1IMZ, where no necrotic neurons were observed. These results indicate that impairment of 123I-IMZ accumulation, namely impairment of 123I-IMZ binding to benzodiazepine receptors, precedes necrotic cellular alterations such as pyknosis, eosinophilia, loss of hematoxylinophilia, scalloping, shrinkage, and swelling. Structural and functional alterations of benzodiazepine receptors, including receptor regulation and transmitter competition, can be potential factors contributing to the altered 123I-IMZ accumulation.
In the ischemic core (region A), accumulation of 123I-IMZ was significantly higher than that of 1-14C-octanoate (Figs. 5C and 5D). The higher accumulation of 123I-IMZ may be ascribed to the destruction of the blood-brain barrier and the increased nonspecific binding of 123I-IMZ, as the lipophilicity of 123I-IMZ is higher than that of 1-14C-octanoate. Another possible explanation for the results is the difference in the times of sacrifice after administration of 123I-IMZ and 1-14C-octanoate. A longer time after administration of the tracer may cause higher accumulation of the tracer in the ischemic core with severely reduced CBF. Matsuda et al. (35) and Toyama et al. (37) compared 123I-IMZ distribution with CBF in rat models of cerebral ischemia and observed a relatively preserved 123I-IMZ accumulation in the ischemic core.
Higher accumulations of 123I-IMP, 123I-IMZ, and 1-14C-octanoate were observed in region B at 24 h than at 4 h. The reasons for the discrepancy remain unclear. The pathophysiology in the region at 24 h may be functionally different from that at 4 h, although necrotic cells were occasionally observed in these regions. The limited number of ROIs assigned to region B may be another reason for the discrepancy. The time points of sacrifice after the ischemic insult were also limited. Further studies, particularly detailed histologic evaluations using a larger number of animals at different time points, are required to clarify the reasons for the discrepancy.
CONCLUSION
The present study demonstrated that 1-14C-octanoate uptake was relatively preserved in the regions without morphologic changes, compared with 123I-IMP and 123I-IMZ uptake. The relatively preserved 1-14C-octanoate accumulation may reflect astroglial function based on fatty acid metabolism. Thus, a possible relationship between the tracer accumulation and the pathologic changes can be summarized as shown in Figure 6. Namely, 123I-IMP accumulation decreases concurrently with CBF after the ischemic insult. 123I-IMZ and 1-14C-octanoate uptake is preserved at the stage with preserved neuronal and astroglial function. 123I-IMZ uptake then decreases with impaired neuronal function based on benzodiazepine receptor binding. Finally, 1-14C-octanoate uptake decreases with impaired astroglial function.
Schematic representation of possible relationship between tracer accumulation and cerebral function. I = preserved neuronal and astroglial function; II = impaired neuronal function and preserved astroglial function; III = impaired neuronal and astroglial function.
The present results provided a useful basis for the development of 1-11C-octanoate as a PET tracer for studying the pathophysiology of ischemic stroke. 1-11C-Octanoate warrants further evaluation as an in vivo indicator of astroglial function based on fatty acid metabolism.
Acknowledgments
This work was supported in part by a grant from the Hokkaido Foundation for the Promotion of Scientific and Industrial Technology. The authors are grateful to Prof. Shinzo Nishi and Prof. Toshiyuki Ohnishi of the Central Institute of Isotope Science, Hokkaido University, for supporting this work.
Footnotes
Received Nov. 4, 2002; revision accepted Mar. 5, 2003.
For correspondence or reprints contact: Yuji Kuge, PhD, Department of Tracer Kinetics, Graduate School of Medicine, Hokkaido University, Kita 15 Nishi 7, Kita-ku, Sapporo 060-8638, Japan.
E-mail: kuge{at}med.hokudai.ac.jp