Abstract
Combined PET/CT imaging has become an integral part of patient management, particularly in oncology. After the imaging examination, a report of the findings is created by expert readers and sent to the referrers as a basis for subsequent decisions. In view of the known wide variation in operational models for PET/CT imaging, we surveyed PET/CT users on their approaches toward PET/CT reporting. Methods: The electronic survey comprised 28 questions on the demographics and professional background of the responders, as well as questions on the structure and quality of PET/CT reports, including the type of reported information, the use of reporting standards, and the mix of reporting standards and expert opinions. The survey was active for 6 wk in early 2018. In total, 242 responses were collected worldwide. Results: The responders were mainly from Europe (78%), with 22% being nuclear medicine specialists, 42% radiologists, 22% dual board-certified, 10% residents in either nuclear medicine or radiology, and 5% medical physicists, radiographers, or oncologists. A slim majority (55%) of responses indicated reports being done according to the European Association of Nuclear Medicine 2015 guidelines for 18F-FDG PET/CT imaging, but 30% of responders were unaware of these guidelines. Report structures varied across sites, with most sites (38%) reporting the PET findings with supplementary localization information from CT, whereas 27% of sites reported along the lines of a CT report with supplementary PET information. One third of the sites included information on the TNM stage of the oncology patient in all reports, whereas 34% and 12% of sites included this information occasionally or only for selected tumors, respectively. For therapy response assessment, various well-established criteria were used. The number of sites utilizing these criteria ranged from 15% (European Organisation for Research and Treatment of Cancer criteria) to 57% (Deauville criteria). Conclusion: Broad variation in the PET/CT reporting strategies adopted for oncology studies and widespread lack of awareness of existing guidelines for PET/CT reporting are evident from responses to this survey, raising concerns as to whether reporting clinicians are optimally using the complementary information from each modality. Greater efforts are needed to ensure harmonization of reporting practices.
See an invited perspective on this article on page 478.
Hybrid imaging methods (1), that is, combinations of complementary anatomic and molecular imaging modalities (PET/CT, SPECT/CT, and PET/MR), have been shown to add significantly to the standard diagnostic work-up of patients by both shortening the acquisition and increasing the diagnostic accuracy over that of stand-alone imaging (2,3). Although evidence supporting these advantages is available from numerous clinical research studies, it is important that this benefit be translated into routine clinical practice. The key vehicle for communicating imaging data to referring clinicians and efficiently integrating it into decision making is the scan report. The value chain of patient management, which includes imaging and reporting, can be described in general terms as follows: patient > attending physician > clinical indication > imaging test > expert reviewing and reporting > attending physician > treatment decision and treatment > follow-up (including imaging) (4). It is obvious that an imaging report (quality, timeliness, communication path) directly influences patient management (5).
Oncologists who refer their patients for a diagnostic work-up with hybrid imaging expect to gain insight not only into the presence of disease but also into its extent and biologic characteristics, as a predictor of disease behavior or as a baseline for assessment of response to treatment. To meet the expectations of referring clinicians, the ultimate product of a high-quality imaging examination should always be a high-quality report that is focused on patient-important parameters with a conclusion aligned with questions pertinent to patient management. In contrast to the wide range of scientific efforts that have been made to optimize hybrid imaging protocols and procedures, comparatively little attention has been given to the quality of the reporting of hybrid imaging examinations, despite early efforts at standardization and harmonization that explicitly included consensus recommendations on the reporting procedures (6–9). Recently, some emphasis has been placed on the use of clinical report structures (10), which may soon become the focus for a new culture of reporting (11).
Despite the importance of the diagnostic imaging report, it is frequently “created with free-style conventional dictation, leading to non-standardized, error prone, vague, incomplete, or untimely delivery of findings with significant interobserver variability.” (12). Moreover, past studies and our own experience show that PET/CT reports, in particular, can vary widely in quality and format, thus potentially leading to misinterpretation and, ultimately, to incorrect patient management (13). Part of the variation in reporting quality and culture may arise from variations in local and professional training programs and backgrounds.
Hybrid imaging modalities, such as PET/CT, stand out from the range of typical radiologic or nuclear medicine imaging examinations. Despite their potential to use synergistic and complementary diagnostic information, existing ownership and training schemes affect the performance of such investigations (14). In light of a growing base of hybrid imaging systems and the potential to increase diagnostic accuracy from combined, “anatometabolic” imaging (15), we intended to probe the current status of PET/CT reporting with regard to oncology patient management. Our key objective was to assess typical reporting regimens in routine clinical use worldwide and to probe the level of standardization and integration of imaging findings with other clinical factors.
MATERIALS AND METHODS
A web-based questionnaire was created by 2 imaging experts, each with over 20 y of experience in PET/CT. The questions, which were in English, related to demographics (e.g., origin of survey responders, their age, their sex, and the nature of PET/CT operations in their practice) and reporting culture (e.g., the structure, content, and dissemination of reports), and the responders were invited to provide anonymized examples of clinical PET/CT reports.
The questionnaire was placed in Google Documents (Appendix A), and on April 10, 2018, an invitation to participate was sent to all registered members of the European Society of Hybrid, Molecular, and Translational Imaging. Approximately 5% of the members of this society are in North America. To further increase potential responses from the United States, a feature article in AuntMinnie on May 2, 2018, provided readers with a link to the survey. In addition, on April 26, 2018, an invitation to partake in a German version of the survey was sent to the members of a German working group on hybrid imaging, a joint venture between the German Society of Nuclear Medicine and the German Radiological Society. Two reminders were sent to the members of the European Society of Hybrid, Molecular and Translational Imaging. The survey was closed on May 28, 2018, thus running for 6 wk.
All responses were checked for completeness and were collected in a Microsoft Excel table. Free-text responses (questions 8, 12, and 23) were collected separately.
RESULTS
Demographics
In total, 242 complete and eligible responses were recorded and available for evaluation. Most of the responses (78%) were from European specialists. The remainder included 11% from Asia, 5% from Africa, 5% from the Americas, and 1% from Oceania (Fig. 1A). Fifty-one responders (22%) were nuclear medicine specialists, 99 (42%) were radiologists, 52 (22%) were dual board-certified, and 22 (10%) were residents in either nuclear medicine or radiology. Of note, 12 (5%) responders were medical physicists, radiographers, or oncologists (Fig. 1B); as far as we are aware, these responders described the reporting situation in their institution but do not do the reporting themselves. One third of the responders were female and two thirds were male, and most were between 35 and 50 y old (Fig. 1C). Their length of experience with PET/CT imaging was 0–1 y (16%), 2–5 y (31%), 6–10 y (26%), 11–15 y (16%), or longer (11%), as indicated in Figure 1D.
Demographic responses (242 eligible responses): responder’s continent of origin (A), professional background (B), age (C), and years of experience with clinical PET/CT (D).
At 44 of the 242 sites (18%), no joint report is generated for clinical PET/CT imaging. Of these 44 sites, 15 (6% of total responses) create separate reports for PET and CT. At 24 sites (10%), no joint assessment or conclusion is available. In the case of separate reports, most are generated by nuclear medicine specialists (39%), radiologists (19%), or dual board-certified readers (16%). In the case of joint PET and CT reports (198/242 sites), most are generated by nuclear medicine physicians (19%), radiologists (9%), both in consensus (52%), or dual board-certified readers (16%). There were no differences in the demographic subgroups, nor were there obvious variations between geographical regions.
The average annual throughput per center is 1,490 ± 50 PET/CT examinations. Overall, 43% of sites reported an annual throughput of 1,000 or less. Most scans (59%) are performed with a low-dose CT protocol. The use of intravenous and oral contrast is frequent (60%–100% of examinations) at 36% and 22% of responding sites, respectively. At 23% of sites, there is a change in the responsibility for reporting, presumably from a nuclear medicine physician to a radiologist, when CT contrast agents are used. There were no differences in the demographic subgroups.
The composition of the reporting teams is shown in Figure 2A. At most sites (96%), reports are made by a team of nuclear medicine experts (nuclear medicine physicians or dual board-certified physicians) and radiology experts (board-certified and residents). At 4% of sites, reports are made by only a radiologist. One site includes a medical oncologist in the reporting team. Representation on tumor boards is shown in Figure 2B. Nuclear medicine physicians present PET/CT findings on 21% of boards; this number increases to 78% if accounting for alternating and consensus presentations or the presence of dual board-certified experts. Again, there were no differences in the demographic subgroups.
(A) Composition of reporting teams for PET/CT studies as percentage of all responses. (B) Tumor board representation as percentage of all responses.
Reporting Culture
Only 55% of sites do their reports according to the 2015 18F-FDG PET/CT guideline of the European Association of Nuclear Medicine (16); in fact, 30% of responders were unaware of these guidelines (Fig. 3A). Of the latter group, 11% are nuclear medicine physicians, 69% are radiologists, and 14% have a dual board certification.
(A) Concordance (% sites) of PET/CT reporting with European Association of Nuclear Medicine guidelines (16). (B) Frequency (% sites) of structured PET/CT reporting. (C) Frequency (% sites) of including prior imaging examinations in reading and reporting of current PET/CT studies.
Most sites (38%) report the PET findings and supplement them with localization information from CT; 27% report the CT findings and supplement them with tracer uptake information from PET (Fig. 3C). Other sites report the findings of both studies: either the CT first and then the PET (11%) or the PET first and then the CT (26%).
Free-text comments were added by 16% of participants. Most of the comments explain in detail how PET and CT data are integrated into one report (e.g., “PET and CT are woven together, and it is clearly stated when PET-findings and CT-findings are described”). Some comments (14/242) relate to completely integrated reporting that differs from combined PET and CT reporting (e.g., a “symbiotic” or “synoptic integrative” report). Half the users always compare current PET/CT images with prior images; 47% do so if prior images are available (Fig. 3B).
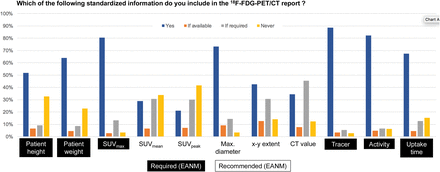
Figure 4 shows the frequencies of a range of quantitative parameters and whether the European Association of Nuclear Medicine requires or recommends that they be reported (16). A significant number of sites do not include recommended information, such as patient weight and height or even the radiotracer. Thirty-two percent of sites include the TNM stage in all oncology reports, whereas 34% and 12% include this information only occasionally or for selected tumors only (Fig. 5A), such as lung cancer, gastrointestinal cancer, and head-and-neck cancer.
(A) Percentage of responders who report TNM stage. (B) Frequency (% sites) of including international criteria in PET/CT reports. “If required” means “if mandated by referring physician.” EORTC = European Organisation for Research and Treatment of Cancer.
When imaging is done to assess the response to therapy, various well-established criteria for measuring the level of disease progression are available to the reporting expert. The number of sites utilizing these criteria ranges from 15% (European Organisation for Research and Treatment of Cancer criteria) to 57% (Deauville criteria (17)) (Fig. 5B).
All results are available on request from the corresponding author.
DISCUSSION
Our survey found a marked heterogeneity in clinical reporting schemes for combined PET/CT imaging. Although most of the reporting teams include a nuclear medicine physician or dual-trained specialist, the actual reporting process varies greatly. Given the range of anatomic imaging protocols used, it is not surprising that the role of the radiologist was less well defined. Our study had 2 key findings on the quality of PET/CT reporting. First, a significant number (18%) of responders indicated that no joint molecular and anatomic imaging report is generated for clinical PET/CT imaging. Second, 30% of the responders were not aware that guidelines for 18F-FDG PET/CT exist (16). Disconcertingly, 45% of the responders do not use these guidelines routinely (Fig. 3A).
Several nonprofit organizations and professional associations have published guidelines on imaging procedures and recommendations for reporting (5,16,18–20), including a list of essential elements in a clinical report (7,21,22). The reporting guidance by Niederkohr et al. from 2013 states specifically that “the accuracy of image interpretation and the quality of the diagnostic report are critical to the continued success of PET/CT in the medical community” (5). This statement should be seen in light of a conclusion drawn from a recent study on malpractice in radiology: “Communication failures are ubiquitous in medicine and lead to a variety of medical errors and patient harm. They are the third most common cause of malpractice against radiologists after diagnostic errors and procedural complications” (23,24). Thus, the quality of the combined nuclear medicine and radiology report is of the utmost importance (25).
In general, communication among physicians is a key factor in health-care delivery. Two aspects of high-quality PET/CT reporting should be considered by every reader. First, the report should cover the essential elements, such as the clinical history, the imaging procedure, the description of comparison studies (if available), the delineation of findings (relevant and incidental), and the final impression (26). Examples of essential report elements for 18F-FDG PET/CT imaging are provided by the Society of Nuclear Medicine and Molecular Imaging PET PROS initiative (18) and in the recommendations by Niederkohr et al. (Table 1 in (5)). Second, the PET/CT report should also integrate the CT data by clearly describing the CT technique, which can be categorized as “low-dose,” referring to nonenhanced CT with reduced tube current settings (used for the purpose of coarse anatomic localization and CT-based attenuation correction), or “high-quality,” referring to a radiologic, diagnostically equivalent CT examination with higher tube output and the use of contrast agents (if applicable) (16). The PET PROS initiative (18) provides the essential elements of PET/CT reporting in detail. According to these guidelines, a report should be structured with station-based findings listed in the craniocaudal direction and should contain a clinically relevant description of the impressions, thereby not deliberately separating the PET findings from the CT findings. An alternative approach is to adopt a TNM-based reporting scheme, which, in our experience, is appreciated by oncologists.
In addition to the structure of the report and the list of essential elements, the choice of reported parameters, such as SUVs and uptake times, may also vary. Our results show a comparatively wide variation in reported quantitative parameters (Fig. 4). The European Association of Nuclear Medicine guideline for 18F-FDG PET/CT imaging, for example, requires readers to report a range of essential parameters (e.g., the radiopharmaceutical and the amount of injected activity in MBq) (16), but our data show that the mandatory parameters are not reported at up to 30% of sites. On the other hand, one study found that some general practitioners do not value inclusion of examination technique or details of the contrast media used (27), and it is possible that oncologists share this view with respect to technical PET/CT details within the imaging report. This possibility might explain why some participants do not follow the guidelines (Fig. 3A).
Nonetheless, because it has been 2 decades since clinical hybrid imaging was introduced, a minimum set of reporting standards should have been accepted by now to support state-of-the-art patient management. By adhering to a high-quality reporting strategy, we can more easily meet the expectations of our referring clinical partners, most of whom prefer to receive standardized reports that consist of templates with separate headings for each organ system (28). Furthermore, Buckley et al. (29) measured the return-call rate after referrers receive unstructured and structured radiology reports and found that a structured report can help the referrer understand the critical findings. According to Brady (11), when nuclear medicine physicians and radiologists are reporting, they should try to put themselves in the shoes of the referrer, thus increasing the likelihood that the meaning of their report and the relevance of their interpretations will be clearly understood.
As a consequence, we see a need to discuss harmonization and standardization of procedures, including reporting. Training in nuclear medicine and radiology should include reporting as well. According to Bosmans et al., 92% of clinicians and 95% of radiologists comment that structured reporting should be an obligatory part of their residency training (28). Our survey had similar findings regarding hybrid imaging. However, hybrid imaging faces many practical and political challenges, since it is driven largely by both nuclear medicine specialists and radiology specialists, whose daily practices and specialty curricula usually do not align well (14). Until these issues are resolved, other professional recommendations by renowned experts may—at least at the local level—be useful on training and reporting practices (26,30,31).
In line with the conclusion from existing but sparse reporting recommendations (7), the hybrid imaging community should strive for PET/CT reports that are consistently of high quality, including a standardized structure and clinically relevant language. The results of this survey may help motivate us to reflect critically on our local practice and potential ways to adapt our imaging reports. As part of such a community effort, it seems reasonable to ask nuclear medicine and radiology associations to work more closely to clarify the existing ambiguities in professional imaging reporting.
Our study had some limitations. First, survey response was voluntary (Appendix A) and, therefore, likely to be biased toward involvement of motivated experts (32). Thus, we assume that the actual heterogeneities of PET/CT reporting regimens are far larger than reported here. However, neither the quality of the sample nor the number of responses can be planned a priori. Second, some responders did not provide eligible answers to every question asked (so-called item nonresponse (33)). This is a problem inherent in survey research itself, regardless of its format or mode of administration. Third, our responses were skewed toward a Eurocentric perspective, given that most of the eligible responses were received from Europe. Finally, our survey did not address in detail all aspects of hybrid imaging reporting. We composed a series of questions that—on the basis of our experience—mirrors the variability of reporting. If adherence to guidelines were to be tested in clinical reality, a regional survey or a survey performed by societal organizations across the globe would be required.
CONCLUSION
A 2018 survey of mainly European PET/CT users demonstrated a marked variation in reporting cultures, with an overall lack of a cross-center standard. Most notably, 18% of sites do not follow a joint PET and CT reporting scheme despite full integration of PET and CT within PET/CT instrumentation. The results from this survey call for a follow-up by professional organizations in an effort to promote high-quality standards in PET/CT reporting.
DISCLOSURE
Rodney Hicks has received travel support from Siemens Healthineers and GE Healthcare and is a recipient of a National Health and Medical Research Foundation of Australia Practitioner Fellowship, which supports his research. Thomas Beyer is a cofounder of cmi-experts GmbH and has received research funding from Siemens Healthineers. Lutz Freudenberg is the owner of 3 private-practice nuclear medicine and radiology departments in Germany and has received speaker funding from Bayer Healthcare. No other potential conflict of interest relevant to this article was reported.
APPENDIX A
Complete List of Questions from the Survey on PET/CT Reporting as Defined in GoogleDocs (http://eshi-society.org/12bqydxn01ky8a/ [password: ESHIsurvey123])
Do you create a joint report for PET/CT examinations?
Who writes the report?
Do you create separate reports for PET and CT?
Is there a joint assessment or conclusion?
Who writes the report?
How many PET/CT examinations does your institution perform per year?
How many % of your PET/CT examinations are performed with a low-dose/full-dose CT?
What factors determine your decision?
How many % of your PET/CT examinations are performed with intravenous contrast agents?
How many % of your PET/CT examinations are performed with oral contrast agents?
Does the responsibility for reporting change when contrast agents are used?
If yes, how?
Who provides the justified indication for .... at your institution?
a. PET
b. CT
c. PET/CT
Who is part of the reporting team?
Who presents PET/CT reports at tumor-boards?
Do you report in accordance with the EANM guideline (FDG-PET/CT)?
Are the individual body regions reported separately (ENT/thorax/abdomen/pelvis/skeleton)?
How is the report structured?
Do you compare current images with prior studies?
Which of the following standardised information do you include?
a. Patient height [cm]
b. Patient weight [kg]
c. SUV max
d. SUV mean
e. SUV peak
f. Max. diameter [mm]
g. x-y extent [mm]
h. CT value [HU]
i. Tracer
j. Amount of radioactivity [MBq]
k. Time of uptake [min]
Which of the following international criteria do you use?
a. EORTC
b. Deauville
c. RECIST
d. PERCIST 1.0
Do you describe the TNM stage?
If “only for certain tumors”, for which ones?
My gender
My place of work is in
My age
My profession
My experience with PET/CT
Acknowledgments
We acknowledge the support of Claudio Brun (European Society of Hybrid, Molecular, and Translational Imaging) and Philip Ward (Aunt Minnie).
Footnotes
Published online Nov. 2, 2018.
- © 2019 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 18, 2018.
- Accepted for publication October 22, 2018.