Visual Abstract
Abstract
Growing interest in PSMA imaging using [68Ga]- or [18F]-labeled ligands and PSMA-based radioligand therapy (RLT) of prostate cancer (PCa) prompted us to survey the global community on their experiences and expectations. Methods: A web-based survey was composed to interrogate areas specific to PET imaging, the clinical value chain, and RLT applications. International responses were collected in early 2022. In total, over 300 valid responses were received and evaluated. Results: Most responses (83%) were given by nuclear medicine specialists with extensive experience in PET. At 22% of sites, PCa ranked “top” in cancer-type–specific PET indications, with an average and median of 15% and 10% of all cases, respectively. The most frequently used PSMA PET tracers were [68Ga]PSMA (32%) and [18F]PSMA-1007 (31%). Users reported a steady growth in PSMA PET and RLT over the past 5 y, averaging 50% and 82%, respectively, with a further 100% median growth projected over the next 5 y. Of note, more respondents indicated cognizance of personalized dosimetry than actually used it routinely. The most commonly identified barriers to future growth in PCa theranostics were radiopharmaceutical supply, reimbursement, staff availability, and buy-in of medical oncologists. Conclusion: Despite enthusiasm, this survey indicates variable adoption of PSMA imaging and RLT globally. Several challenges need to be addressed by the medical community, authorities, and patient advocacy groups in integrating PSMA-targeted theranostics into personalized medicine.
Theranostics, diagnostic radiopharmaceuticals guiding radioligand therapy (RLT) (1), has long been embedded in nuclear medicine in the form of radioiodine imaging and therapy. Whole-body evaluation of target expression using PET of highly specific tracers identifies patients likely to benefit from RLT (2). Theranostics paradigms have generated broad interest among health-care providers (3,4) and led to substantial investments by the pharmaceutical industry.
RLT targeting the prostate-specific membrane antigen (PSMA RLT) has attracted particular attention (5). Diagnostically, this theranostic paradigm has been facilitated by the emergence of PSMA PET ligands that enable highly sensitive detection of metastatic disease (6), which is a major cause of morbidity and premature death from prostate cancer (PCa) that has become castration-resistant (7). In prospective studies, PSMA PET is superior to conventional staging procedures at both initial diagnosis (8,9) and in the setting of biochemical recurrence (BCR) (10) and has been included in the recently revised National Comprehensive Cancer Network (NCCN) guidelines and approved for reimbursement by U.S. Medicare. Accordingly, PSMA-based PET, combined predominantly with CT (PET/CT) but also, due to more restricted availability, with MRI (PET/MRI), is becoming a standard of care for the evaluation of high-risk PCa (11,12).
The high PSMA expression in most metastatic, castration-resistant prostate cancer (mCRPCa) has encouraged the development of agents with suitable pharmacokinetics for RLT, typically labeled with 177Lu but also with other radionuclides including the α-particle emitter 225Ac (13). Prospective trials have demonstrated safety and efficacy in heavily pretreated and often refractory disease settings (14,15) and have been further reinforced by the results of the TheraP (16) and the more recent VISION (17) trials. These studies have recently led to regulatory approval of PSMA-617 (Pluvicto; Novartis) by the Food and Drug Administration (FDA), representing the first targeted RLT for treatment of progressive, PSMA-positive mCRPCa. With FDA approval of [68Ga]-PSMA-11, which is now available as a cold kit formulation (Illuccix; Telix), and [18F]-Pyl, both diagnostic and therapeutic arms are now accessible for clinical use.
As these studies have captured the attention of the urologic community (18–20), the authors felt it was timely to investigate the status of PCa theranostics globally and to assess perceptions regarding future evolution and define barriers to its broader clinical application.
MATERIALS AND METHODS
Survey
The survey comprised 46 questions, including multiple-choice and free-text formats (Supplemental Appendix 1; supplemental materials are available at http://jnm.snmjournals.org) prepared in SurveyMonkey (https://www.surveymonkey.de/r/psma-pet_2022) and launched in early 2022 via an email distribution list by the German Association of Nuclear Medicine (DGN) and European Association of Nuclear Medicine (EANM) Newsletters in early January. The invitation to participate in the survey came with introductory text explaining the objective of this survey and seeking participation. The survey invitation was extended to key contacts in Australia and, at the suggestion of the International Atomic Energy Agency (IAEA), to Japan, Korea, South Africa, and Chile later in January. Invitees were asked to disseminate the link among their professional networks. The survey was closed in late February without reminders being issued. All data entries were stored in xls-format and transferred to the authors for data analysis.
Survey Structure
The survey had 5 categories: Section A (General PET imaging) detailing routine PET experience; Section B (Prostate cancer–specific PET imaging) interrogating PSMA PET; Section C (Reporting and clinical value chain) surveying operational aspects of diagnostic work-up of PCa; Section D (Theranostics and dosimetry), regarding integrated theranostics; and, finally, Section E (Demographics), collecting information regarding the respondent’s professional background and geographical location.
Data, Data Entry, and Extraction
Overall, 384 independent surveys were submitted. As this survey was targeted at an audience familiar with, and active in, providing PSMA PET imaging in routine clinical scenarios, we assumed that either questions 6 (Annual PET studies for PCa) and 7 (Annual PET studies for all other cancers), or question 8 (Fraction of annual PET for PCa?) should have elicited a positive response (Supplemental Appendix 1). Forty-five entries did not contain that information and were removed before analysis. Further, multiple entries from the same site were reduced to 1 entry per site using a random number generator to deselect 11 of 22 entries with identical IP addresses. On the basis of these assumptions, 328 completed forms were analyzed.
Reporting Results
We report average values of numeric entries per category and present key results in graphical format. Entries that permitted for free-text entry were summarized in commentaries highlighting key phrases.
RESULTS
Demographics (Fig. 1)
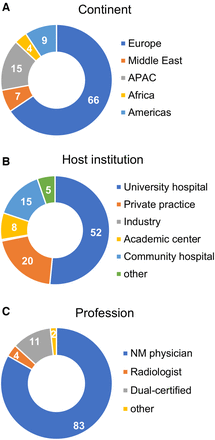
The highest number of valid responses was from Europe (66%) followed by Asia-Pacific (APAC) (15%) and the Americas (9%). About half of all responses originated in university hospitals (52%); the remainder were from private practice (20%), academic centers (8%), community hospitals (15%), and other (5%). Over 80% of respondents were nuclear medicine specialists, with 11% from dual-certified specialists, 4% from radiologists, and 2% from others (medical physicists, technologists, radiopharmacists).
Demographics: most valid responses came from Europe (66%) (A), originated from university hospitals (52%) (B), and were given by nuclear medicine (NM) specialists (83%) (C).
General PET Imaging
Three hundred thirteen of 328 (95%) of respondents were actively involved in clinical PET, with up to 35 y (mean ± SD, 14 ±7 y; median, 7 y) of experience. An overwhelming majority (322/328, 98%) indicated oncology to be the primary focus of their program, with key indications being staging (51%), restaging (30%), and therapy monitoring (19%).
At the time of the survey, sites were acquiring an average of 3,000 PET scans per year (maximum, 25,000) with 15 ±15% for PCa evaluation. PET throughput for PCa was variable but most sites reported acquiring fewer than 300 Pca PET scans per year (Fig. 2). On average, sites obtained 15 ±14% of their annual PET workload for PCa diagnosis (range [0–100]%), with 22% sites reporting this to be the highest cancer-specific clinical indication, whereas 39% reported it to be lowest tier.
(A) Logarithmic display of annual PET scan numbers for PCa versus all PET; each dot represents 1 reporting site. (B) Most sites (105) obtain up to 100 scans for PCa per year with only very few (18) sites obtaining more than 1,000 PET scans for PCa. The number of PET scanners per site was unknown.
Prostate Cancer and PET Imaging
Of those using PSMA PET imaging routinely, one quarter each used it for staging and BCR. Between 13% and 16% of sites used PSMA PET for patient selection for RLT and for monitoring response to systemic therapy (Fig. 3A). A very small number of sites (4%) reported other-use cases, such as increase of prostate-specific antigen levels greater than 20% and follow-up after curative intent radiotherapy (21).
(A) Key indications for PSMA-PET imaging in [%]sites. (B) Use of PET imaging tracer portfolio across valid responses. dx = diagnosed; pats = patients; pls = please.
Sites used mainly [68Ga]PSMA ligands (32%) and [18F]-PSMA-1007 (31%), with the remainder using [18F]choline (7%) and [18F]DCFPyl (2%). Only 1 site was routinely using fluciclovine. Less than 9% reported use of other tracers or combinations thereof. Unfortunately, 19% reported no tracer specifically, even though they claimed a significant portion of PET scans being obtained for PCa patients. A small number of sites (7%) reported additional use of [18F]FDG PET, whereas 26% never perform separate [18F]FDG examinations; 41% and 26% reported an add-on [18F]FDG scan to be obtained rarely or sometimes, respectively.
One third of the users stated that they did not have sufficient PET imaging capacity to address patient needs; the remainder reported sufficient capacity. Figure 4A illustrates the wide range of waiting times across responding sites (1 to 120 d, with a mean of 17 ± 16 d; median, 10 d). Of note, the median waiting time varied considerably between 7 and 21 d across geographic regions, with the longest waiting time at sites in Africa (Fig. 4B).
(A) Typical waiting times for prostate cancer patients to undergo a PSMA PET imaging examination across all responding sites (valid responses only). Waiting times range from 1 to 120 d, with a median of 10 d. (B) Median waiting times varied across continents between 7 d (APAC and Middle East) and 21 d (Africa).
Most (60%) respondents reported a change in tracer use over the past 5 y. This included a shift from [18F]-choline to PSMA (both, 68Ga- and 18F-labeled), the adoption of [18F]PSMA-1007, and occasional use of [99mTc]PSMA (4 sites). Three sites reported a change from [18F]-labeled PSMA to [68Ga]PSMA.
Growth rates over the past year were estimated to be between 10% (Europe) and 25% (Americas) for PET imaging in general and similar for PSMA PET imaging, with the exception of higher growth in the Middle East and Africa (30% each). Midterm growth of PSMA PET imaging is anticipated to increase substantially: Europe (50%), Middle East (45%), APAC (60%), Africa (100%), and Americas (85%).
Reporting and Clinical Value Chain
About 50% of respondents stated that reporting times for PSMA PET were similar to those of [18F]FDG PET, whereas 45% and 5% stated it to be shorter and longer, respectively. For more complex situations, such as for treatment selection and response assessment, 44% stated that reporting time did not change significantly, whereas 45% and 11% reported on moderately and significantly increased times, respectively. The key factors leading to increased reporting times included “unspecific PSMA expression in bone,” “unspecific uptake in lymph nodes,” “inflammatory processes,” “interpreting low grade uptake in prostate s/p treatment,” and “liver metastases.” Additionally, teaching gaps (“using new guidelines on reporting on PSMA PET”) and structural inefficiencies (“lack of clinical information”) were described.
Standardized reporting is used by 37% sites, whereas 30% plan to do so shortly; the remaining third (32%) do not use standardized reporting. When standardized reporting is used, 49% suggested the use of a local, in-house standard, whereas 51% used published standards, most frequently: PROMISE (22) and E-PSMA (23). Very few responders referred to the use of miRADS criteria (24). With respect to CT acquisition parameters of the PSMA PET/CT imaging protocols, most sites (77%) use a low-dose CT only, whereas only 15% of the sites reported the use of contrast-enhanced CT in urogram phase as part of their standard CT acquisition protocol.
Most responses on capacity limitations centered on expansion of imaging infrastructure (PET/CT and PET/MRI), radiopharmaceutical availability, and adequate staffing (physicians, technologists, or radiographers) to meet demands. For RLT, access to 177Lu-PSMA was a constraint. Of note, 1 response said “… By far the most important [requirement] will be the education of referring physicians about PSMA PET/CT imaging.”
Participants in this survey appeared hesitant to adopt 99mTc-PSMA, considering it appropriate only for image-guided radiosurgery and requiring wider availability at lower costs.
Theranostics and Dosimetry
Forty-eight percent of respondents performed 177Lu-PSMA RLT. Of these, 44% believed personalized dosimetry to be potentially “valuable,” but only one third (36%) of these respondents actually do it, with 20% thinking it not useful and 36% not being sure. Only 1 site reported that RLT would be adjusted if advised by the dosimetry calculation.
Figure 5 shows that of sites providing RLT, most used 177Lu-PSMA-617 (52%) or 177Lu-PSMA-I&T (31%). A small fraction also reported using 225Ac-labeled PSMA-617 (10%) and I&T (7%). There was variable throughput across sites, with an average of 134 ± 333 cycles per year (median, 50). One site each in South Africa, India, and Germany reported 1,000 or more therapy cycles annually. Figure 6 indicates that anticipated short-term growth rates of PSMA-based therapies were more variable than midterm growth rates, which are expected to double.
Reported frequencies (%) of radiopharmaceuticals for RLT.
Median growth rates (%) for Lu-PSMA RLT for 2021 (−1 y) and 2023 (+1 y) across responses from 5 continental regions. Growth rates were expected highest for Africa and Middle East.
Close to 60% sites receive PSMA RLT agents from external suppliers, the remainder compounding on-site. On average, two thirds of the respondents performed PSMA-based therapy for a VISION- (17) or TheraP- (16) like population, a quarter for mCRPCa patients before chemotherapy, and 20% each for castration-sensitive metastatic disease and other indications (Fig. 7). The medians were 43%, 14%, 25%, and 25%, respectively. Almost all theranostic users performed posttreatment imaging (85%). When done, 38% used that information to abbreviate treatment and 18% for treatment extension, but 42% were “not sure” why. Most responses (60%) stated that there will likely be other radionuclides, particularly α-emitting 225Ac, that will compete clinically with 177Lu.
Key indications for 177Lu-PSMA therapies at sites engaged in RLT: (category 1) VISION- or TheraP-like population, (category 2) castration-resistant patient prechemotherapy, (category 3) castration-sensitive metastatic disease, and other. The majority (42%) caters to VISION trial– or TheraP-like populations (blue bars, category 1).
Regarding key challenges to clinical theranostics in PCa, most responses commented on the high costs of the radiopharmaceuticals and limited reimbursement. Equally important, people voiced concerns over their interaction with oncologists and urologists (e.g., “referral by oncologists who prefer multiple lines of chemo,” “attitude of the oncologists,” “Integration with oncologist and urologist in the indications and management of patients,” “Convince medical oncologists to refer the cases earlier than after trying multiple chemotherapies,” “…When we treat when patient is almost bed ridden the results are not better and we cannot assess the survival”). There was frequent mention of the need for broader education and training. Another frequently highlighted challenge is that of “heterogeneous response” of patients and that of “toxicity” of treatment. Less frequently mentioned challenges included the paucity of randomized controlled trials, the wish for combination therapies, the need for clearer instructions on patient selection for RLT, industry support, and capacity building.
DISCUSSION
This survey gathered a significant number of responses from around the globe. Most responses were provided by nuclear medicine specialists who were primarily based at academic centers, particularly in Europe (Fig. 1), and generally from well-established clinical PET programs (Fig. 2). With favorable compassionate access regulations allowing use of these PSMA ligands in Germany and Australia in particular, the current responses are likely to have been somewhat biased by “early adopters” of these agents. Experience elsewhere, particularly the broader European and North American nuclear medicine communities, for which access only became more recently available primarily through industry-sponsored trials, may be less well represented.
Despite this potential limitation, this survey provides a useful snapshot of the current status of PSMA-based theranostics globally and, importantly, suggests high expectations of growth (Fig. 6). This optimistic perspective notwithstanding, the surveyed parties identified the need for increasing staffing resources, wider reimbursement, the adoption of standardized protocols, and expanded training—to name a few—as challenges confronting their clinical practices.
The most frequent indications for PSMA PET (Fig. 3A) were BCR and initial staging, primarily for the detection of otherwise occult metastatic disease in intermediate- to high-risk primary disease rather than diagnosis per se. The tracers reported to be most routinely in use were [18F]-PSMA-1007 (25) and [68Ga]PSMA-11 (26) (Fig. 3B). Despite being available in several European jurisdictions for some time before access to PSMA ligands, choline-based PET (7%) has been largely replaced by PSMA due to evidence of enhanced sensitivity (27). Interestingly, only 1.5% of the surveyed sites reported the use of [18F]-DCFPyL (28), which is not yet widely available in Europe. After FDA approval, DCFPyl is now finding penetration in North America and Australia. In Europe, the ability to source [18F]-labeled agents made under good-manufacturing-practice conditions may have affected the pattern of use of PSMA-1007 versus [68Ga]-PSMA-11 and I&T. The reduced urinary excretion of PSMA-1007 has been promoted as one of the advantages of this agent over other PSMA ligands, but this is somewhat offset by higher liver parenchyma activity, potentially limiting detection of hepatic metastases, and concerns regarding its specificity for bone metastasis (29). Irrespective of the PET tracer in use, waiting times for patients were on the order of 10 to 20 d (range, 1–120) (Fig. 4A).
Respondents recognized a need for approved indications of PSMA imaging to be broadened, especially for therapeutic guidance in advanced PCa, including selection of patients for PSMA RLT, which, in turn, would necessitate a corresponding increase in imaging capability. This also could imply an increasing role for [99mTc]-labeled PSMA tracers, which are currently mainly advocated for radioguided surgery (30). The focus on use of PSMA imaging for treatment selection was, perhaps, driven by the observation that about half of the responses came from sites with comprehensive theranostics programs.
When considering issues of service delivery, these were again probably influenced by the nature of nuclear medicine practice in Europe, where PET and therapeutic nuclear medicine are substantially delivered by physicians in hospitals with academic affiliations rather than by radiologists working in the private sector. This may account for contrast-enhanced (ceCT) in urogram phase being performed at only 15% of sites surveyed and standard contrast-enhanced ceCT in just over half of sites. By differentiating between ureteric and adjacent nodal uptake (31), ceCT in the urogram phase is more likely to assist the interpretation of [68Ga]PSMA-11 or [18F]-DCFPyL PET images for the higher renal excretion of these tracers than that with [18F]-PSMA-1007. Further, delayed CT imaging after urinary clearance may, however, assist with such agents. These nuances of CT protocols were not further interrogated in this survey. Neither was query done with regard to the use of PET/CT versus PET/MR in bespoke clinical indications. It is anticipated that as PSMA PET/CT and PSMA PET/MRI become more widely available in the United States, where most nuclear medicine specialists are also radiologists, and RLT is also adopted by radiation oncologists, the patterns of use and imaging protocols adopted may well change. A follow-up survey will be useful to document changing practice.
Considering therapy, growth rates in Lu-PSMA RLT over the short term were highest in the Americas (>60%), possibly because they are coming off a relatively low base with previously limited but now expanding accessibility (Fig. 6). Annualized growth is expected to stay as high as 100% over the next 5 y, with slight variations across the globe. Nonetheless, to sustain such impressive growth, even if estimated solely from throughput numbers on site, costs for RLT must be brought down or effectively buffered by reimbursement schemes in countries with high gross domestic products and universal health-care coverage. Barriers to reimbursement were perceived to include the generation of prospective data demonstrating the efficacy of theranostic approaches in impacting PCa management and improving patient-important outcomes. Further obstacles in full implementation of PSMA theranostics were noted to be a need for broader training and education as well as a closer and respectful cooperation with urologists and oncologists (32).
Although almost half of the responding sites (48%) commented that they are currently performing Lu-PSMA therapy onsite, the use of posttreatment dosimetry for adjusting administered activity appears to be limited. Just over 40% thought of personalized dosimetry as being of great use. However, only one third (36%) of those who do perform Lu-PSMA therapy and indicated personalized dosimetry as being important, performed it routinely (Fig. 5A). The reasons for this could not be deduced from this survey.
Surveys are generally limited by the relatively small response rates. Here, the rate was estimated to be around 3% of the size of the targeted participants. Although low, this is similar to surveys conducted previously by some of the contributing authors (33,34). As noted above, there seems to have been a predilection for responses being received from sites with established and relatively high-volume PET programs in academic centers in Europe and with substantial experience already in PSMA theranostics. Nevertheless, the experience and perspectives of these early adopters is likely to be helpful in informing the broader nuclear medicine community and new entrants into the field. Should this survey be repeated in 2–3 y, urologists and radiation oncology experts should be included as a target audience given their rising interest in engaging in the application of therapeutic radioligands. In this regard, the nuclear medicine community is encouraged to continue considering publication of original research and reviews in urology and oncology journals to increase awareness and broaden education of both providers and users of these technologies.
CONCLUSION
This international survey attests wide global interest in PET-based diagnosis and RLT for PCa. Currently, most sites use [18F]-PSMA-1007 or [68Ga]PSMA-11 for BCR and staging primary PCa. Respondents expected a median growth in PSMA PET imaging of 50%–100% across the globe. Half of the responses came from sites that also engage in RLT, but many fewer sites use dosimetry. Results from this survey call for capacity building and engagement of urologists and oncologists while ensuring wider availability of radiopharmaceuticals at lower costs and with broader reimbursement.
DISCLOSURE
Thomas Beyer is cofounder of cmi-experts GmbH and Dedicaid GmbH and recipient of research grants from Siemens Healthineers. Frederik L. Giesel is codeveloper of PSMA-1007 and advisor to ABX, Telix Pharmaceuticals, and SOFIE; he has a patent application for quinolone-based fibroblast activation protein–targeting agents for imaging and therapy in nuclear medicine and shares in iTheranostics. Rodney J Hicks holds shares in Telix, is an advisor to Revela Pharmaceuticals and is founder, board chair and CMO of PreMIT Pty Ltd., which receives research grant funding from Siemens Healthineers. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the current global status of PSMA theranostics in management of prostate cancer?
PERTINENT FINDINGS: With some regional variations, PET imaging and PSMA-based RLT are gaining momentum globally but face several obstacles to widespread accessibility.
IMPLICATIONS FOR PATIENT CARE: Capacity building, training, user-friendly quantitative imaging, and improved treatment protocols to address heterogeneity of response are required for sustained growth of PSMA-theranostics.
ACKNOWLEDGMENTS
We gratefully acknowledge the advice of Michael Hofman during the design of the questionnaire. We thank Tobias Fabiunke and Goetz Jonas (DGN Office) for implementation of the survey and its curation, and Henryk Silver (EANM Office) for support of the dissemination of the survey to the EANM community. Dissemination across the Swiss and Austrian Nuclear Medicine community was supported by John Prior (SGNM) and Petra Neubauer and Hans-Jürgen Gallowitsch (OGNBM), respectively. We much appreciate the advice from Diana Paez, Andrew Scott Jun Hatazawa, Henry Bom, Mike Sathekge, and Horacio Amaral on wider distribution of this survey.
Footnotes
Guest Editor: Todd Peterson, Vanderbilt University.
Published online Aug. 11, 2022.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 21, 2022.
- Revision received June 29, 2022.