Visual Abstract
Abstract
177Lu-labeled prostate-specific membrane antigen (PSMA) radioligand therapy effectively treats metastatic castration-resistant prostate cancer. Patients requiring treatment, and consequently the number of theranostic centers, are expected to increase significantly after Food and Drug Administration and European Medicines Agency approval. This requires standardization or harmonization among theranostic centers. The aim of this study was to assess operational differences and similarities among 177Lu-PSMA treatment centers. Methods: A questionnaire comprising 62 items, designed by a core team of 5 physicians and externally reviewed by international experts, was developed. Study participants were asked to provide answers about their center, patient selection, radiopharmaceuticals, clinical assessment before and after 177Lu-PSMA treatments, laboratory values, treatment discontinuation, posttreatment imaging, and general information. An invitation e-mail to participate in the study was sent in June 2022. Duplicates were removed to allow for only one valid response per center. Results: Ninety-five of 211 (45%) contacted centers completed the questionnaire. Most participating centers were in Europe (51%), followed by America (22%) and Asia (22%). During the 12 mo before this study, a total of 5,906 patients received 177Lu-PSMA therapy at the 95 participating centers. Most of these patients were treated in Europe (2,840/5,906; 48%), followed by Asia (1,313/5,906; 22%) and Oceania (1,225/5,906; 21%). PSMA PET eligibility for 177Lu-PSMA was determined most frequently using 68Ga-PSMA-11 (77%). Additional pretherapy imaging included 18F-FDG PET/CT, CT, renal scintigraphy, and bone scintigraphy at 41 (49%), 27 (32%), 25 (30%), and 13 (15%), respectively, of the 84 centers for clinical standard of care, compassionate care, or local research protocols and 11 (26%), 25 (60%), 9 (21%), and 28 (67%), respectively, of the 42 centers for industry-sponsored trials. PSMA PET eligibility criteria included subjective qualitative assessment of PSMA positivity at 33% of centers, VISION criteria at 23%, and TheraP criteria at 13%. The mean standard injected activity per cycle was 7.3 GBq (range, 5.5–11.1 GBq). Sixty-two (65%) centers applied standardized response assessment criteria, and PSMA PET Progression Criteria were the most applied (37%). Conclusion: Results from this international survey revealed interinstitutional differences in several aspects of 177Lu-PSMA radionuclide therapy, including patient selection, administered activity, and the response assessment strategy. Standardization or harmonization of protocols and dedicated training are desirable in anticipation of increasing numbers of patients and theranostic centers.
Prospective single-arm (1–3), randomized (4,5) clinical trials have shown 177Lu-labeled prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) to be effective for treating metastatic castration-resistant prostate cancer (CRPC). It received U.S Food and Drug Administration and European Medicines Agency approval in 2022 and was swiftly adopted into prostate cancer management guidelines (6–8). PSMA PET imaging and 177Lu-PSMA RLT are gaining momentum globally, but treatment delivery has faced several obstacles limiting its widespread accessibility. Patient numbers are expected to increase significantly, with an estimated 34,000 prostate cancer patients requiring approximately 120,000 177Lu-PSMA treatment cycles per year in the United States alone (9,10). Approximately 140 U.S. centers are needed to satisfy this demand, assuming the administration of 4 cycles per center per day (9).
Besides the limited number of treatment centers, challenges include radiopharmaceutical production and delivery, with demand–supply imbalance, lack of medical provider training and competence, and the need for additional workforce, including nuclear medicine physicians and nursing staff (11). Despite a widely varying regulatory, financial, and medical landscape, the nuclear medicine community has been spearheading efforts to meet the need for this new standard-of-care (SOC) treatment (12). Joint guidelines were recently proposed by the European Association of Nuclear Medicine, Society of Nuclear Medicine and Molecular Imaging, and International Atomic Energy Agency to establish an overarching framework helping practitioners understand what is required to set up a theranostics center (13,14). Further guidance is provided by joint procedure guidelines of the European Association of Nuclear Medicine and Society of Nuclear Medicine and Molecular Imaging (15). Moreover, the nuclear medicine community has engaged with urooncology experts to incorporate PSMA imaging and radionuclide therapy into clinical practice consensus guidelines (16,17).
177Lu-vipivotide tetraxetan, also known as 177Lu-PSMA-617, is Food and Drug Administration–approved for treatment of adults with PSMA-positive metastatic CRPC who have been treated with androgen receptor pathway inhibitor and taxane-based chemotherapy. The prescribing information suggests patient selection based on PSMA imaging and the administration of 7.4 GBq (200 mCi) every 6 wk for up to 6 doses, as the registrational VISION trial proposed (4). However, criteria defining 177Lu-PSMA RLT eligibility by PSMA PET, therapy protocols, therapy response assessment, and parameters for treatment discontinuation all differ between established theranostic centers and countries. Therefore, the aim of this international questionnaire study was to assess operational differences and similarities between 177Lu-PSMA treatment centers.
MATERIALS AND METHODS
The survey comprised 62 questions including multiple-choice and free-text answers and was prepared using Qualtrics XM in a web-based design (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). The questions were drafted by UCLA investigators and externally reviewed by 5 international experts in the field of PSMA theranostics. Once the final version of the questionnaire was outlined, an official invitation e-mail to participate in the study was sent in June 2022. The invitation was sent to all centers involved in patient recruitment for the TheraP and VISION trials (4,5), the corresponding authors on clinical 177Lu-PSMA publications (screened through PubMed), and international contacts of the investigators. Duplicates were removed to allow for only one valid response per center. The survey was closed in late September 2022.
Survey Structure
The questionnaire involved general physician and center-specific questions; questions on patient selection, radiopharmaceuticals, clinical assessment before and after 177Lu-PSMA treatments, laboratory values, treatment discontinuation, and posttreatment imaging; and general questions (Supplemental Fig. 1).
Data Analysis
Survey answers were exported in an Excel (Microsoft) spreadsheet, and the data were analyzed. Descriptive analysis was performed using SPSS software (IBM).
RESULTS
Geographic Location of Participating Centers
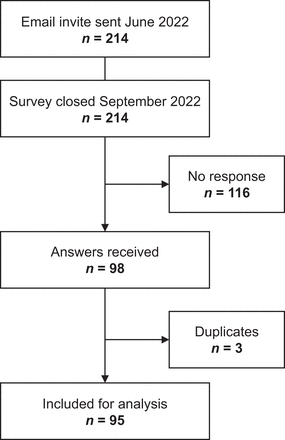
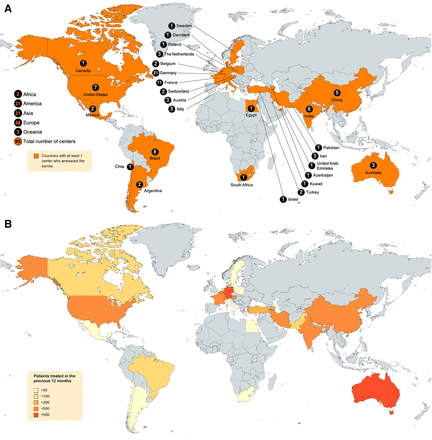
In total, 95 of 211 (45%) contacted centers completed the questionnaire (Fig. 1). Most participating centers were in Europe (48; 51%), followed by North and South America (21; 22%), Asia (21; 22%), Oceania (3; 3%), and Africa (2; 2%). On a national level, Germany (22%), France (12%), Brazil (8%), the United States (7%), India (6%), and China (5%) provided the highest number of participating centers (Fig. 2A).
Diagram of number of participating centers.
Geographic location of participating centers (A), and heat map of number of patients treated between June 2021 and September 2022 (B).
Population Characteristics
During the 12 mo before the study, a total of 5,906 patients received 177Lu-PSMA therapy at the 95 participating centers. Most patients were treated in Europe (2,840/5,906; 48%), followed by Asia (1,313/5,906; 22%) and Oceania (1,225/5,906; 21%) (Fig. 2B). Most centers were actively involved in 177Lu-PSMA through different models of care: 177Lu-PSMA was given at 84 (88%) centers as SOC treatment or compassionate-care access (CCA), at 42 (44%) centers as part of industry-sponsored clinical trials, and at 21 (22%) centers as part of locally approved research protocols (LARPs) not sponsored by industry (multiple options of care possible per center; therefore, number exceeds 100%; Fig. 3; Supplemental Fig. 2). Forty-six (48%) centers treated patients only with metastatic CRPC, whereas 47 (49%) centers treated patients with metastatic CRPC and hormone-sensitive prostate cancer (HSPC). Two (2%) centers treated only HSPC.
177Lu-PSMA model of care among participating centers (multiple answers allowed).
Initiation of PSMA RLT
Ten (11%) centers started PSMA RLT before 2015, 64 (67%) between 2015 and 2020, and 21 (22%) between 2021 and 2022. The earliest 131I-MIP-1095 had been used was 2011. Overall, 50% of centers were already treating patients before 2018. Supplemental Figure 3 shows increments of PSMA RLT centers per continent.
Pretreatment Imaging and PSMA PET Eligibility Criteria
Pretreatment PSMA Imaging
PSMA PET or PSMA SPECT was performed at all participating centers to assess patient eligibility for 177Lu-PSMA RLT (Fig. 4). 68Ga-PSMA-11 was the most frequently used PET radiotracer (73; 77%), followed by 18F-PSMA-1007 (39; 41%), 68Ga-PSMA-I&T (21; 22%), and 18F-DCFPyL (18; 19%) (continent-based analysis in Supplemental Fig. 4). At 12 (13%) centers, 99mTc-labeled PSMA for SPECT imaging was sufficient to assess 177Lu-PSMA RLT eligibility, and these locations were predominantly in Germany (5/12), Iran (2/12), and Mexico (2/12).
Imaging modalities performed to assess patient eligibility classified by model of care (multiple answers allowed).
Additional Pretreatment Imaging
18F-FDG PET/CT was performed at 49% of centers when 177Lu-PSMA therapy was provided as SOC, CCA, or LARP not sponsored by industry and at 26% of centers when patients were enrolled in industry-sponsored clinical trials (Fig. 4). Additional pretherapy imaging included CT (SOC + CCA + LARP, 32%; industry-sponsored trials, 60%), bone scintigraphy (SOC + CCA + LARP, 15%; industry-sponsored trials, 67%), renal scintigraphy (SOC + CCA + LARP, 30%; industry-sponsored trials, 21%), and others (Fig. 4). Geographic differences were evident mainly for pretherapy renal scintigraphy (e.g., as part of the eligibility process at 15 of 21 centers in Germany) and for choline PET (e.g., performed at 9 of 11 centers in France).
PSMA PET Eligibility Criteria
The most frequently applied PSMA PET eligibility criterion for 177Lu-PSMA RLT was a subjective visual whole-body tumor PSMA positivity evaluation (33%), followed by assessment of tumor PSMA uptake in comparison to liver (defined as >50% of tumor lesions with uptake more than in the liver) (26%), VISION criteria (23%), and TheraP criteria (13%) (Fig. 5). No significant differences among continents were observed for applied eligibility criteria.
PSMA PET eligibility criteria for 177Lu-PSMA RLT.
Performance Status and Quality of Life
To be eligible for 177Lu-PSMA RLT, patients had to have an Eastern Cooperative Oncology Group performance status no higher than 1 at 2 centers (2%), 2 at 65 (68%), 3 at 22 (23%), and 4 at 6 (6%). Pretreatment quality-of-life assessment using validated questionnaires was not performed routinely at 67 (71%) centers. Among quality-of-life tools, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 was the most commonly used, that is, at 14 (15%) centers.
Treatment
Administered Radiopharmaceuticals
For RLT agents, 48 (51%) centers use 177Lu PSMA-617 only, 21 (22%) 177Lu PSMA-I&T only, and 26 (27%) both 177Lu PSMA-617 and 177Lu PSMA-I&T. Additionally, 7 (7%) were also using other labeled PSMA-targeting agents such as 225Ac-PSMA.
Therapy Dose and Interval Between Treatment Cycles
Mean standard injected radioactivity per cycle for 177Lu-PSMA RLT was 7.3 GBq (range, 5.5–11.1 GBq). Continent-based subanalysis showed an average injected radioactivity (GBq) per cycle of 7.5 ± 0.1, 7.3 ± 0.4, 7.5 ± 1.1, 7.1 ± 0.7, and 8.2 ± 0.3 for Africa, America, Asia, Europe, and Oceania, respectively. Dose deescalation was performed at 10 (11%) centers. Injected activity was adapted on the basis of bone marrow, salivary gland, kidney, or liver function at 50 (53%) centers; the patients’ PSMA-positive tumor volume at 12 (13%); patient weight at 9 (9%); and dosimetry measurements at 6 (6%).
The most frequent intervals between 177Lu-PSMA RLT cycles was 6 wk at 57 centers (60%) and 8 wk at 26 (27%). Six (7%) centers adapted the intervals between cycles on the basis of prostate-specific antigen (PSA) levels and clinical parameters.
Response Assessment
Imaging Response Criteria
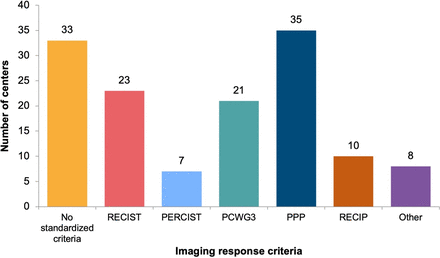
The PSMA PET Progression Criteria were most frequently applied (35; 37%), followed by RECIST 1.1 (23; 24%), the Prostate Cancer Working Group Criteria (PCWG3) (21; 22%), the Response Evaluation Criteria in Prostate Cancer (RECIP) 1.0 (10; 11%), and PERCIST (7; 7%) (Fig. 6; Supplemental Fig. 5). Multiple answers were allowed for this question. Thirty-three (35%) centers did not apply standardized radiographic criteria for response assessment.
Imaging response criteria for 177Lu-PSMA (multiple answers allowed). PPP = PSMA PET Progression Criteria.
Timing of Radiographic Response Assessment
PSMA PET was performed for response assessment at 83 (87%) centers. PSMA PET was performed before the third treatment cycle at 48 (51%) centers and after completion of therapy at 63 (66%) centers. Twenty-four (25%) centers indicated that the timing of imaging response assessment was variable, depending on biochemical parameters and clinical status.
Clinical Assessment During Therapy
Parameters systematically evaluated by most (≥80%) centers throughout the course of 177Lu-PSMA RLT included pain, fatigue, xerostomia, appetite, weight, and quality of life, at 99%, 95%, 88%, 85%, 83%, and 83% of centers, respectively.
Laboratory Parameters
Blood was drawn most frequently 2 and 3 wk after each therapy cycle at 45 (47%) and 20 (21%) centers, respectively. Blood was drawn most frequently 1 and 2 wk before the next therapy cycle at 62 (65%) and 24 (25%) centers, respectively.
At least 98% of centers measured platelets, erythrocytes, hemoglobin, and total white cell count before treatment initiation and between cycles. Some discrepancies were found between the blood tests requested at treatment initiation and between treatment cycles, including PSA (99% vs. 94%), neutrophils (92% vs. 83%), creatinine (99% vs. 91%), glomerular filtration rate (87% vs. 73%), liver enzymes (94% vs 82%), alkaline phosphatase (92% vs. 77%), and lactate dehydrogenase (74% vs. 57%) (Fig. 7).
Laboratory parameters assessed before and between 177Lu-PSMA RLT cycles. AST/ALT = aspartate transaminase/alanine transaminase; GFR = glomerular filtration rate; Hb = hemoglobin; LDH = lactate dehydrogenase.
177Lu-PSMA γ-Imaging
Posttreatment 177Lu-PSMA γ-imaging was performed at 90 (95%) of centers. Regarding each treatment cycle, 94% of centers performed 177Lu-PSMA γ-imaging after the first cycle, 87% after the second, and 85% after the third and fourth cycles. Whole-body planar acquisition was most frequently used (77%), followed by semiquantitative SPECT with 2 or more bed positions (37%). The time of 177Lu-PSMA γ-image acquisition was 4, 24, 48, and 72 h after injection at 18%, 62%, 32%, and 12% of centers, respectively. Ten (11%) stated that they always acquire images at at least 2 different time points.
Discontinuation
Treatment discontinuation was more frequent after the second treatment cycle (54; 57%) than after the third treatment cycle (37; 39%). A rising PSA required confirmation before 177Lu-PSMA RLT discontinuation, with a second PSA sample obtained at 14 (15%) centers, whereas confirmatory imaging in conjunction with a second PSA sample was a requirement for discontinuation at 65 (68%) centers. Parameters leading alone to treatment discontinuation were low platelets at 61 centers (64%), low neutrophils at 58 (61%), and low hemoglobin at 31 (33%). A rise in PSA alone led to treatment discontinuation at 21 (22%) centers.
Role of Nuclear Medicine Physician
The responders were nuclear medicine physicians at 88 (93%) centers, medical oncologists at 2 (2%), radiation oncologists at 2 (2%), and others at 3 (3%) (1 radiologist, 1 internal medicine physician, and 1 radiochemist). At 94 (99%) centers, nuclear medicine physicians were involved in at least one aspect of 177Lu-PSMA RLT, namely evaluation of the treatment indication (89; 94%), assessment of patient eligibility (86; 91%), and management between cycles (87; 92%). Patients were followed up for posttherapy outcomes by nuclear medicine physicians at 74% of centers. At 23 (24%) centers, the nuclear medicine physician was not involved in the discussion of treatment discontinuation. At the 72 (76%) centers where the nuclear medicine physician was involved in the discussion of treatment discontinuation, it was the nuclear medicine physician’s responsibility to bring the treatment discontinuation discussion to a multidisciplinary team at 65 of 72 (90%) centers.
177Lu-PSMA RLT Reimbursement
177Lu-PSMA RLT was completely covered by the health care system at 51 (54%) centers, whereas 19 (20%) centers reported only partial coverage. No insurance coverage was reported at 25 (26%) centers.
177Lu-PSMA RLT was performed as an outpatient procedure at 46 (48%) centers and as a 1-, 2-, and 3-d inpatient procedure at 19 (20%), 16 (17%), and 14 (15%) centers, respectively (Supplemental Fig. 6).
DISCUSSION
The rapid increase in use of theranostics is being addressed by scientific societies supporting theranostic centers with treatment guidelines (13–15,18). The current study demonstrated interinstitutional differences in 177Lu-PSMA RLT operations, including patient selection, dosing, response assessment, and treatment discontinuation. In part, these differences reflect variations in accepted standards of practice as reflected in guidelines and the evidence base. The survey also, however, identifies some areas of concern. Although data on the effectiveness of 177Lu-PSMA RLT in HSPC are still lacking, 49% of participating centers were treating both CRPC and HSPC, which is remarkably higher than was found in a previous survey reporting 20% of centers treating CRPC and HSPC (19). This difference may, in part, reflect increased participation in trials investigating the effectiveness of 177Lu-PSMA RLT in HSPC, such as the LuTectomy (NCT04430192), UpFrontPSMA (NCT04343885), and PSMAddition (NCT04720157) trials. However, sufficient data are still missing to entirely justify 177Lu-PSMA RLT in HSPC patient outside prospective studies.
Interestingly, at 68% of centers, an Eastern Cooperative Oncology Group score of 2 was the highest performance status accepted for 177Lu-PSMA therapy, whereas 29% of centers accepted an Eastern Cooperative Oncology Group score of up to 3 or 4. This might reflect differences in the training and competence of physician and nonphysician staff in providing appropriate care to patients with poor functional status.
The survey highlights variations in patient selection. The Food and Drug Administration advised that selection of patients for treatment using an approved PSMA imaging agent be based on PSMA expression in tumors. Several PSMA PET eligibility criteria have been proposed, including criteria published in reports of previous prospective trials (4,5). However, we found that, most commonly, a subjective visual whole-body tumor PSMA positivity evaluation (33%) was performed to assess treatment eligibility. In several studies investigating predictive PSMA PET imaging for assessing response to 177Lu-PSMA RLT, the biomarkers differed from those in previously published prospective trials and require further investigation (20–22).
Even though the mean standard injected activity per cycle for 177Lu-PSMA RLT was almost similar to what is recommended by the Food and Drug Administration (7.3 vs. 7.4 GBq), the administered doses in our survey ranged between 5.5 and 11.1 GBq. More than half the centers (53%) adapted the injected dose on the basis of the patient’s bone marrow, salivary gland, kidney, or liver function. Further trials are needed to better understand the morbidity and mortality of adapted versus fixed-dose protocols. Also, dose deescalation or adaptation based on patient weight or dosimetry was performed at fewer than 12% of centers.
In the European Association of Urology–European Association of Nuclear Medicine Consensus Statement, the experts achieved consensus on the use of PSMA PET/CT in the evaluation of response to 177Lu-PSMA RLT even though no consensus on the timing of PSMA PET/CT was reached (23). This reflects the heterogeneity of our findings, with centers applying PET at different intervals after treatment. The panelists discussed neither the specific radiographic response criteria nor the possibility of assessing treatment response using posttreatment 177Lu-PSMA γ-imaging, a method that around 30% of centers participating in our survey use. In this context, the PROMISE V2 guidelines also provide a framework for response criteria (24). In metastatic prostate cancer, treatment response was traditionally evaluated using CT/MRI and bone scanning according to the PCWG3 criteria (25). Nevertheless, neither PCWG3 nor RECIST 1.1 (26) or PERCIST 1.0 (27) was designed to include PSMA PET/CT imaging. The PSMA PET Progression Criteria (28) and RECIP 1.0 (29) were just recently introduced but had already been applied at 37% and 11% of participating centers, respectively. A recent study investigated the accuracy of RECIST 1.1, adapted PCWG3, adapted PERCIST 1.0, the PSMA PET Progression Criteria, and RECIP 1.0 for response evaluation using PSMA PET/CT in men with metastatic CRPC treated with 177Lu-PSMA RLT (30). Among the assessed frameworks, RECIP 1.0 were found to have the highest prognostic value and interreader reliability. However, one third (35%) of centers do not apply any standardized imaging criteria for therapy response evaluation. RECIP originally integrated software-based quantitative assessment of total tumor volume (quantitative RECIP), but wide clinical implementation of such software is not expected soon. Recently, RECIP—determined using visual reads by nuclear medicine physicians (visual RECIP)—showed 95% agreement with quantitative RECIP (31). Hence, RECIP can immediately be implemented in daily practice.
Since a large number of metastatic CRPC patients might not benefit from 177Lu-PSMA RLT (1–3), criteria for treatment discontinuation need to be discussed. Our results suggest that the clinical complexity does not allow for easy establishment of criteria or definite cutoffs. There is agreement that a single post–177Lu-PSMA RLT PSA increase alone is not sufficient to justify 177Lu-PSMA RLT discontinuation but that confirmation by a second PSA sample or radiologic progression is required.
All participating centers stated that PSMA imaging, that is, PET or SPECT, was mandatory to assess 177Lu-PSMA RLT eligibility. On the basis of the European Association of Urology–European Association of Nuclear Medicine consensus statement, PSMA PET/CT should be performed on any candidate before 177Lu-PSMA RLT (23), whereas in our results 13% of centers affirmed that they also use PSMA SPECT imaging. Evaluation of PSMA expression is crucial to assess 177Lu-PSMA RLT eligibility, but limiting evaluation of PSMA receptor expression to PET imaging might potentially exclude countries and centers without established access to PET imaging. Use of additional pretreatment imaging was variable, especially among patients participating in sponsored trials versus protocols outside industry-sponsored trials, including SOC. Patients participating in industry-sponsored trials were more likely to undergo additional bone scanning and CT, whereas protocols outside industry-sponsored trials more often included 18F-FDG PET/CT to assess patient eligibility. These differences might be explained by the use of the PCWG3 criteria for response assessment in clinical trials.
The need for a robust supply chain for 177Lu-PSMA is crucial to meet the increasing demand for 177Lu-PSMA RLT (9). A previous study highlighted that 5% of the patients died while waiting for a supply of 177Lu-PSMA (32).
This study had several limitations. First, a high number of responses were received from Europe, given the early use and high number of treatment centers. Second, countries with a small number of centers but high patient volumes might be underrepresented since the data do not account for the number of treated patients per center. Third, Food and Drug Administration approval of 177Lu-PSMA in 2022 in part changed the practice of U.S. centers from treating patients as part of research protocols to SOC. Fourth, national regulatory differences impact several aspects of 177Lu-PSMA RLT.
CONCLUSION
Results from this international survey revealed significant interinstitutional differences regarding multiple aspects of 177Lu-PSMA RLT, such as eligibility assessment, administered activity, and response assessment strategies. In part, this variation reflects differences in accepted practice standards supported by evolving clinical practice guidelines. Some responses, however, raise concern and highlight the need for theranostic centers, specific training, and an improved evidence base as theranostics is widely adopted.
DISCLOSURE
Andrea Farolfi reports fees from Telix (speaker) and Calyx (image review). Wesley Armstrong is supported by the UCLA–Caltech Medical Scientist Training Program (NIGMS T32 GM008042). Lena Unterrainer reports fees from Astellas (speaker) and Novartis (consultant, speaker) outside the submitted work. Michael Hofman acknowledges philanthropic/government grant support from the Prostate Cancer Foundation (PCF) funded by Canica, the Peter MacCallum Foundation, the Medical Research Future Fund (MRFF), an NHMRC investigator grant, Movember, and the Prostate Cancer Foundation of Australia (PCFA); acknowledges research grant support (to the institution) from Novartis (including AAA and Endocyte), ANSTO, Bayer, Isotopia, and MIM; and declares personal consulting fees for lectures or advisory boards from Astellas and AstraZeneca in the last 2 y. Matthias Eiber reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant, speaker), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Eckert-Ziegler (speaker), Janssen Pharmaceuticals (consultant, speakers bureau), Parexel (image review), and Bioclinica (image review) outside the submitted work and a patent application for rhPSMA. Wolfgang Fendler reports fees from SOFIE Biosciences (research funding), Janssen (consultant, speaker), Calyx (consultant, image review), Bayer (consultant, speaker, research funding), Novartis (speaker, consultant), Telix (speaker), GE Healthcare (speaker), and Eczacıbaşı Monrol (speaker) outside the submitted work. Boris Hadaschik reports the following: advisory board (Janssen, Bayer, ABX, Lightpoint, Amgen, MSD, Pfizer, Novartis), invited speaker (Astellas, Janssen R&D), institutional royalties (Uromed), institutional funding (AAA/Novartis, BMS, German Research Foundation), an advisory role (German Cancer Aid), and a leadership role/speaker (DKG AUO). Ken Herrmann reports personal fees from Bayer, personal fees and other from SOFIE Biosciences, personal fees from SIRTEX, nonfinancial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Fusion, personal fees from Immedica, personal fees from Onkowissen.de, personal fees from Novartis, personal fees from ymabs, personal fees from Aktis Oncology, personal fees from Theragnostics, personal fees from Pharma15, personal fees from Debiopharm, personal fees from AstraZeneca, and personal fees from Janssen. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How is 177Lu-PSMA RLT organized around the world?
PERTINENT FINDINGS: A questionnaire was developed, and 95 theranostic centers around the globe answered. The aim was to assess operational differences and similarities between 177Lu-PSMA treatment centers. We found significant interinstitutional differences regarding multiple aspects of 177Lu-PSMA RLT, such as eligibility assessment, administered activity, and response assessment strategies.
IMPLICATIONS FOR PATIENT CARE: There is a need for specific training and an improved evidence base because theranostics is being widely adopted.
Footnotes
Guest Editor: David A. Mankoff, University of Pennsylvania
Published online Jan. 18, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication July 19, 2023.
- Accepted for publication November 18, 2023.