Visual Abstract
Abstract
177Lu can be imaged after administration using SPECT/CT. Most work to date has focused on using posttreatment imaging to measure normal organ and tumor dose. We aimed to assess the impact of posttreatment SPECT/CT on the management of patients undergoing 177Lu-prostate-specific membrane antigen (PSMA) radiopharmaceutical therapy (RPT). Methods: In this retrospective study, 122 patients underwent PSMA RPT with subsequent SPECT/CT 24 h after treatment. We determined a qualitative response at each cycle and reviewed patient charts to assess the impact that posttreatment SPECT/CT had on patient management. Changes in patient management were classified as changes on the basis of progression and response, and specific cycles when they occurred were noted. Miscellaneous changes in patient management were also evaluated. Results: Among the 122 consecutive patients examined, 42%–56% exhibited stable disease, whereas 19%–39% of patients exhibited response on visual assessment across treatment cycles. In total, 49% (n = 60) of patients experienced changes in management, of which 57% (n = 34) were due to progression, 40% (n = 24) were due to response, and 3% (n = 2) were due to miscellaneous changes. Changes due to disease progression were observed mostly after cycles 2 and 4. Changes due to response to RPT occurred mostly after cycles 3 and 4. Conclusion: At our center, 49% of patients experienced changes in management based on posttreatment SPECT/CT, and most of these changes occurred at cycles 2 and 4. Integrating posttreatment SPECT/CT into routine PSMA RPT protocols can aid in patient management.
- radiopharmaceutical therapy
- 177Lu-PSMA-617
- metastatic castration-resistant prostate cancer
- posttreatment SPECT/CT
Prostate-specific membrane antigen (PSMA) radiopharmaceutical therapy (RPT) using 177Lu-PSMA-617 (Pluvicto; Novartis) has been shown to prolong overall survival in patients with metastatic castration-resistant prostate cancer after chemotherapy in the VISION trial (1–5). The current treatment paradigm is to treat patients every 6 wk with 7.4 GBq (200 mCi) of 177Lu-PSMA-617 (6).
One of the struggles with PSMA RPT is how to decide when to stop treatment. Treatment decisions take into account clinical response, tumor response using imaging, and laboratory studies consisting of serum prostate-specific antigen (PSA) levels. One particularly difficult issue is imaging tumor response. For nodal disease and soft-tissue lesions, CT can be useful to measure morphologic changes, but most patients have osseous disease which is not well evaluated using CT or bone scans (6,7). In clinical trials, there are strict rules about when treatment continues or stops. In the VISION trial, treatment was stopped only at the time of progression on the basis of the Prostate Cancer Working Group 3 criteria (1,8). Additionally, for osseous disease, the Prostate Cancer Working Group 3 criteria require bone scans, although in clinical practice, they are not routinely used.
177Lu emits multiple γ-photons, which can be directly imaged using SPECT/CT, allowing one to qualitatively visualize the distribution of the treatment and to perform quantitative dosimetry (9–12). Qualitative evaluation of posttreatment SPECT in the TheraP trial was used to stop treatment early if patients achieved a complete response (13). Other work has shown that the response on SPECT/CT correlates with improved overall survival or progression-free survival (14–16). Although delaying clinical treatment using posttreatment 24-h SPECT/CT is one facet being explored (17), other crucial aspects such as treatment cessation due to disease progression or response, elucidating the impact of SPECT/CT on patient management, are yet to be delineated.
At our institution, we have performed posttreatment SPECT/CT on all patients undergoing PSMA RPT. In this work, our aim is to understand the impact of posttreatment SPECT/CT on management changes in patients treated with 177Lu-PSMA-617 RPT.
MATERIALS AND METHODS
Study Population
We performed a retrospective review of patients who received 177Lu-PSMA-617 RPT at our institution as the standard clinical care and through the expanded access program from November 2021 to February 2024. Patients who had received a minimum of 2 cycles of PSMA RPT and had a posttreatment SPECT/CT performed after each cycle were included. Morphologic imaging (CT) was performed as a part of our institutional protocol, and patients were imaged after cycles 2, 4, and 6. Patients had their serum PSA levels checked the day of treatment and between each cycle. Follow-up of patients was continued until time of death to determine overall survival. The institutional review board of UCSF approved this retrospective study, and the requirement to obtain informed consent was waived.
SPECT/CT Scan Acquisition
Most patients were imaged using whole-body planar and SPECT/CT 1 d after each cycle in the context of routine clinical care using a dual-head γ-camera (Infinia Hawkeye; GE Healthcare) system with the following acquisition parameters: 208% ± 10% keV photopeak, 170% ± 10% keV scatter window, 128 × 128 matrix, 30 s per projection, 60 projections in total using 2 detectors, medium energy general-purpose collimators, and a low-dose CT for attenuation correction. An Xeleris workstation (GE Healthcare) was used for reconstruction with the following reconstruction parameters: ordered-subset expectation maximization, 10 iterations, 6 subsets, and a Butterworth filter, with scatter correction and attenuation correction. The imaging duration was approximately 60 min, consisting of 1 whole-body planar acquisition and 2 20-min SPECT/CT bed positions covering the kidneys and most of the tumors. A subset of patients were imaged with a cadmium zinc telluride–based digital SPECT/CT (Starguide; GE Healthcare), with the following acquisition parameters: 208% ± 6% keV photopeak, 185% ± 5% keV scatter window, 3 min per bed position with 7 to 8 beds for a total SPECT acquisition time of 21–24 min, 272 × 272 matrix, 2.46 mm voxel size, and a diagnostic CT without contrast.
Qualitative Response Assessment
The SPECT/CT performed after cycle 1 served as a baseline, and subsequent time points were qualitatively analyzed by 2 nuclear medicine physicians to assess response to PSMA RPT. Response at each cycle was determined relative to the prior cycle and not relative to the baseline SPECT/CT. Because of the absence of an established framework for SPECT lesion-based response, we adapted the response biomarker-guided approach by Emmett et al. (17) to assess the response using SPECT and the accompanying diagnostic CT images. Qualitative response assessment was divided into 3 categories: progression (>30% increase in tumor volume with or without new disease sites or new PSMA-negative lesions), response (>30% decrease in tumor volume with no new tumor lesions, PSMA positive or negative), and stable disease (does not meet criteria for progression or response).
PSA and Posttreatment SPECT/CT Relationship
A change in PSA of greater than 25% was used to define an increase or decrease in PSA, which was adapted from the Prostate Cancer Working Group 3 criteria (8). The change in PSA at each cycle was compared with the corresponding imaging response, which resulted in 5 groups: PSA response after each cycle mirrored the corresponding posttreatment SPECT/CT response; SPECT/CT showed stable disease with decreased PSA; SPECT/CT showed stable disease and with increased PSA; SPECT/CT showed progression with decreased PSA; SPECT/CT showed a response with increased PSA.
Characterization of Management Changes
Patient clinical charts, including notes from the treating nuclear medicine physician and medical oncologist, were assessed to determine whether posttreatment SPECT/CT after each cycle resulted in a change in patient management. Patients whose posttreatment SPECT/CT led to management changes were further examined, and changes that led to early discontinuation of PSMA RPT (before 6 cycles) were divided into changes based on progression and response. Progression changes included progressive disease that led to discontinuation of PSMA RPT. Additionally, these were subcategorized as those that were confirmed subsequently on conventional imaging and those that were not. Response changes included evidence of response to PSMA RPT that led to stopping treatment early. Within the response category, patients for whom a lower threshold was used to stop treatment in the setting of development of hematologic toxicities were categorized. Additionally, a third category of miscellaneous patient management changes included situations in which SPECT/CT helped address specific issues like hydronephrosis and back pain.
Statistical Analysis
Quantitative variables from the clinical data were described using descriptive statistics in the form of the median and interquartile range for continuous variables and count and percentage for binary variables. We assessed the decline in PSA from baseline of at least 50% at any given time during the treatment (8). For cases in which more than 1 type of change in patient management was observed, only the initial change in management was considered for analysis to prevent duplication of patient data. We used the Kaplan–Meier method to compare the outcomes in patients who stopped early for response and stopped early for progression. Additionally, correlation between PSA and SPECT/CT responses with overall survival among the 5 response groups was performed.
RESULTS
Patient Characteristics
In total, 141 patients underwent 177Lu-PSMA-617 RPT at our institution between November 2021 and February 2024. A total of 124 patients received 2 or more cycles, of which 2 patients did not receive SPECT/CT. Patients received a median of 4 cycles (interquartile range, 3–5 cycles), and 17 patients are continuing treatment. Of 122 patients, 81 (66%) experienced a response showing a decline in PSA from baseline of at least 50% (Table 1; Supplemental Fig. 1).
Patient Characteristics
Qualitative Response Assessment
The predominant imaging response observed was stable disease, noted in 42%–56% of posttreatment SPECT/CT scans depending on the cycle of PSMA RPT (Table 2). Response to PSMA RPT was seen in 19%–39% of patients. Most patients achieved a response by cycles 2 (39%) and 3 (30%). Progression was seen in 14%–39% of patients, depending on the treatment cycle.
Qualitative Response Assessment on Posttreatment SPECT/CT After Each PSMA RPT Cycle
PSA, Posttreatment SPECT/CT, and Outcomes
The PSA response after each cycle mirrored the corresponding posttreatment SPECT/CT response in 44% (54/122) of patients. Of 122 patients, 68 (56%) showed discordant SPECT/CT and PSA responses. Of the 68 patients that showed discordance, 29% (20/68) had more than 1 instance of discordance noted over their cycles. SPECT/CT showed stable disease with decreased PSA in 63% (43/68) of patients. SPECT/CT showed stable disease with increased PSA in 46% (31/68) of patients. In 12% (8/68) of patients, PSA decreased while SPECT/CT showed progression. In 10% (7/68) of patients, SPECT/CT showed a response with increased PSA. Notably, group 4, in which SPECT/CT showed progression despite a decrease in PSA and treatment that was stopped early, had the lowest median overall survival of 198 d although the CIs overlapped (Supplemental Table 1).
Changes in Patient Management
Posttreatment SPECT/CT prompted a change in management in 49% (60/122) of patients (Table 3). Of those, in 97% (58/60) of patients, PSMA RPT was stopped early based on posttreatment imaging: 34 patients had a change based on progression, and 24 patients had a change based on response. In 2 (3%) patients, posttreatment imaging led to miscellaneous changes, including placement of a nephrostomy tube for hydronephrosis, and localizing lumbar pain that was subsequently treated with stereotactic body radiation therapy (Table 3).
Types of Change in Patient Management
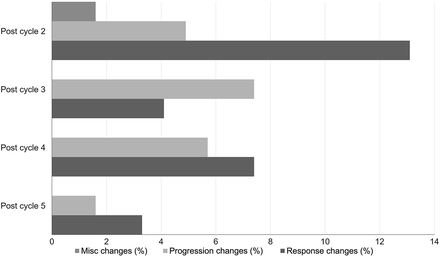
Among the 34 patients with progression changes, 28 stopped RPT because of progressive disease seen on SPECT/CT, whereas in 6 patients, progression detected on posttreatment SPECT/CT was confirmed on morphologic imaging before discontinuation (Table 3). Of these 34 patients, most exhibited a change in management at cycle 2 (47%; 16/34) and cycle 4 (26%; 9/34) (Fig. 1). Additionally, it was noted that although the posttreatment SPECT/CT showed progression, the PSA decreased or was stable in 26% (9/34) of patients, with most discordance occurring after cycle 2 (7/9). Of these 9 patients, 2 converted to chemotherapy, 2 to immunotherapy, 1 to enzalutamide, 1 to radiation therapy, 1 to 223Ra therapy, and 2 died of disease-related complications before initiating alternative treatment.
Percentage of patients at each cycle that had change in management based on posttreatment SPECT/CT, broken down by cycle and type of management change. Misc = miscellaneous.
Among the 24 patients with response changes, 20 stopped RPT because of the response on SPECT/CT, whereas in 4 patients, RPT was stopped because of partial response on posttreatment SPECT/CT in the setting of hematologic toxicities (Table 3). Of these 24 patients, most exhibited a change in management after cycle 3 (38%; 9/24) and cycle 4 (29%; 7/24) (Fig. 1). Patients who stopped treatment because of response demonstrated improved survival outcomes compared with those who stopped because of progression (P < 0.0001; Supplemental Fig. 2).
Case Examples
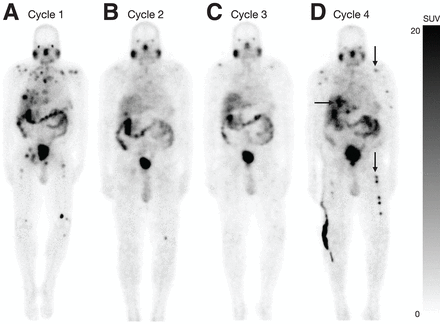
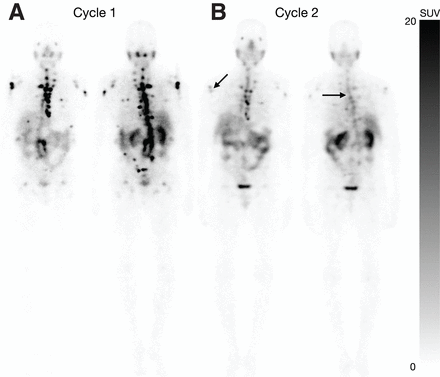
Case 1 demonstrates a 72-y-old man who stopped treatment after 4 cycles because of disease progression on posttreatment SPECT/CT (Fig. 2). The second case demonstrates a 70-y-old man who showed marked response after cycle 2 SPECT/CT, leading to stoppage of treatment. The patient had a PSA decline of 90% from baseline after cycle 2 and continues to be PSA progression free after 4 mo of stopping treatment (Fig. 3). The third case demonstrates a 74-y-old man who stopped the treatment after 4 cycles because of disease progression in the liver even though the osseous lesions showed a response on SPECT/CT after cycle 4 (Fig. 4). The fourth case demonstrates a 68-y-old man who stopped treatment after 2 cycles because of suspected progression in the liver based on maximum-intensity projection images that were confirmed on SPECT/CT and subsequent conventional imaging (Fig. 5). The fifth case demonstrates a 71-y-old man who stopped treatment after 4 cycles because of the response on posttreatment SPECT/CT in the setting of anemia and thrombocytopenia (Supplemental Fig. 3).
Image of 72-y-old man demonstrates change in patient management because of progression, with cessation of PSMA RPT after 4 cycles. (A) Planar imaging after cycle 1 demonstrates uptake in osseous and hepatic disease. (B and C) Whole-body maximum-intensity projection images after cycle 2 (B) and after cycle 3 (C) demonstrate response to treatment with decrease in previously visualized disease. (D) Maximum-intensity projection image after cycle 4 shows increase in osseous and hepatic disease (arrows). PSA levels were stable up to cycle 3, and then increased from 20 to 82 ng/mL at cycle 4. Cycle 5 was not administered because of evidence of progression, and patient was shifted to hospice care.
Image of 70-y-old man demonstrates change in patient management because of marked response, resulting in early stopping of PSMA RPT. (A) Planar imaging after cycle 1 demonstrates uptake in osseous and nodal disease. (B) Planar imaging after cycle 2 demonstrates marked reduction in uptake in previously visualized disease (arrows), which mirrored PSA decline of 99% by cycle 2. Treatment was stopped because of marked response, and PSA had not yet progressed 7 mo after stopping treatment.
Image of 74-y-old man demonstrates change in patient management based on progression, resulting in stopping of PSMA RPT. (A) Planar imaging after cycle 1 demonstrates uptake in osseous and hepatic disease. (B–D) Planar imaging after cycle 2 (B) and after cycle 3 (C) demonstrates gradual increase in liver lesion (arrows) and reduction of uptake in existing osseous disease. (D) Development of new lesions in distal lower extremities (dotted arrow). Liver lesion continued to increase in size after cycle 4 SPECT/CT (solid arrow). Although there was partial response in osseous lesions, cycle 5 was not administered because of evidence of progression in liver lesions, and patient was converted to treatment with chemotherapy.
Image of 68-y-old man demonstrates change in patient management because of progression in liver lesions, leading to stopping after 2 cycles. (A) Maximum-intensity projection image after cycle 1. (B) SPECT/CT image demonstrates no significant uptake in liver after cycle 2. (C and D) Maximum-intensity projection image (C) shows increase in number of liver lesions (dotted circle) which is confirmed on SPECT/CT (D, arrow). (E and F) Follow-up contrast-enhanced CT (CECT) demonstrates increase in liver disease (F, arrow) compared with CECT prior to cycle 1 (E).
DISCUSSION
We demonstrated that posttreatment SPECT/CT changed the management in 49% of patients undergoing PSMA RPT. Most progression changes leading to discontinuation occurred after cycles 2 and 4, whereas most response changes leading to early treatment cessation occurred after cycles 3 and 4.
Prior research has primarily focused on leveraging posttreatment SPECT/CT for evaluating dose–response relationships (10,12,18,19). More recently, the potential of SPECT parameters such as SUV and total tumor volume to evaluate response has been investigated (14,15,20).
In clinical settings, response assessments and decisions on discontinuing PSMA RPT are primarily based on clinical judgment, PSA response, and findings on either morphologic imaging or PSMA PET. A recent international survey comparing operational RPT protocols in 95 centers around the world found that although posttreatment SPECT is conducted at most centers (90%), it is used for response evaluation in only 30% (21).
Previous studies have shown that stopping PSMA RPT early on the basis of PSA levels and imaging response might be possible for patients who respond well (13,17). In our previous work evaluating outcomes of PSMA RPT, 19% of patients stopped treatment early on the basis of PSA response and posttreatment SPECT/CT (22). Aligning with these findings, we noted response changes with early cessation of PSMA RPT in 20% (26/122) of patients. Additionally, many metastatic castration-resistant prostate cancer patients do not respond to PSMA RPT (2,3), and our results demonstrate that posttreatment SPECT/CT can be effective in identifying these patients as early as 6 wk into treatment. In our study, between 9% and 13% of patients stopped the treatment after each cycle because of progressive disease.
Although obtaining baseline SPECT/CT after the first cycle is essential for comparison with subsequent SPECT/CTs, the role of scans after cycle 6 remains unclear. As retreatment with PSMA RPT is used more frequently, scans after cycle 6 may serve as a fresh baseline for PSMA-avid disease in patients screened for retreatment. Lastly, although we observed changes in management, since considerable variations exist in standards of practice (21), further research is crucial to make clear guidelines and cutoff values for managing patients who respond or progress on PSMA RPT.
Another important issue in monitoring response to PSMA RPT is the dissociation between PSA and PSMA imaging in a subset of patients (15,20). We found that in 26% of patients with progression changes, imaging progression preceded PSA progression. This is typically due to the development of new sites of disease while baseline PSMA-avid disease is still responding. The appropriate change in management in patients who have evidence of progression on posttreatment imaging and whose PSA is decreasing is unclear. In our center, in general, changes on SPECT/CT overruled changes in PSA. In the 9 patients for whom PSA decreased while tumor volume increased, treatment was stopped early and patients were switched to a different therapy. Interestingly, all of these discrepancies occurred after cycle 2.
Posttreatment SPECT/CT can be used for 2 purposes: to conduct dosimetry and to perform response assessment. Although there is a significant interest in using quantitative dosimetry in PSMA RPT (12,18,23), its impact on treatment decisions remains unclear because of the absence of a consensus on normal organ dose limits and tumor-absorbed dose targets. Research focusing on quantifying posttreatment SPECT/CT using SUVs and tumor volumes is ongoing (14,15). This approach is valuable, especially in extensive bone disease in which a change in tumor volume may be a better indicator of response assessment than intensity (24). Our results highlight the value of visual assessment of posttreatment SPECT/CT for identifying patients who require treatment modifications based on progression or response to PSMA RPT. Visual analysis is not only rapid but also easily implemented in clinical settings. Moreover, collecting posttreatment scans for qualitative assessment enables data accumulation for exploring the role of quantitative SPECT/CT and dosimetry.
Response assessment to PSMA RPT remains largely unanswered. PSA’s inherent variability in metastatic prostate cancer limits its effectiveness as a sole indicator of response to PSMA RPT (8,25,26), particularly in the early treatment cycles. Interim PSMA PET has been proposed (27–29), but there is not a standardized recommendation regarding the cycle for obtaining it. However, with SPECT/CT imaging, it is feasible to perform posttreatment SPECT after every cycle at a significantly lower cost compared with a single PSMA PET scan. Moreover, contemporary SPECT/CT scanners have been found to deliver quantitative imaging parameters comparable to PSMA PET (14). It should be remembered that contrast-enhanced CT is still required for evaluating PSMA-negative disease during treatment, particularly in the liver. Recently, the utility of posttreatment SPECT/CT as a response assessment tool is also under exploration (14,17).
In our practice, we have selected 24-h posttreatment SPECT/CT to image patients. This was selected by weighing the demand on our patients having to come back for additional imaging and the increased quantitative accuracy with more delayed imaging. Imaging beyond 1 d would require our patients to stay in the region for multiple days and limit the days on which we can provide treatment. Imaging the day of has been shown to be feasible (30), but earlier time points are less quantitatively accurate for dosimetry (10,19).
This study has several important limitations. It is a retrospective single-center study, and it is important to note that clinical decisions may vary between centers. The determination of a change in patient management was based retrospectively on chart review, which may be less accurate and differ from prospective determination of changes in patient management. Additionally, the criteria for assessing response on SPECT/CT scans were qualitative, and quantitative volume measurements were not performed. It should be noted that previous work using PSMA PET demonstrated near equivalence of qualitative and quantitative evaluation of response (29). Despite these limitations, our study suggests the potential benefits of including posttreatment SPECT/CT in the response assessment algorithm for PSMA RPT.
CONCLUSION
In this retrospective study, posttreatment SPECT/CT informed changes in management in 49% of patients with changes based on progression occurring mostly after cycles 2 and 4 and changes based on response occurring mostly after cycles 3 and 4. Further work aiming at standardization of posttreatment imaging is warranted.
DISCLOSURE
Thomas Hope has grant funding to the institution from Clovis Oncology, GE Healthcare, Lantheus, Janssen, the Prostate Cancer Foundation, Telix, and the National Cancer Institute (R01CA235741 and R01CA212148). He received personal fees from Bayer, BlueEarth Diagnostics, and Lantheus and received fees from and has an equity interest in Curium. Robert Flavell has received prior research funding from Bristol Meyers-Squibb, Fibrogen, and Fukushima SiC, not related to this research. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How often does posttreatment SPECT/CT impact patient management during 177Lu-PSMA RPT?
PERTINENT FINDINGS: Qualitative posttreatment SPECT/CT triggered a change in management in 49% of patients undergoing PSMA RPT, with changes based on progression occurring mostly after cycles 2 and 4 and changes based on response occurring mostly after cycles 3 and 4.
IMPLICATIONS FOR PATIENT CARE: Integrating posttreatment SPECT/CT into the routine PSMA RPT workflow can streamline personalized patient management by serving as a response marker to RPT.
Footnotes
Published online Aug. 8, 2024.
- © 2024 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication April 15, 2024.
- Accepted for publication July 4, 2024.