Abstract
The aim of this study was to evaluate the diagnostic value of a new somatostatin analog, 99mTc-P829, compared with that of 111In-pentetreotide. Methods: Forty-three patients (32 men, 11 women; age range, 24–78 y; mean age, 56 y) with biologically or histologically proven neuroendocrine tumors were prospectively included: 11 patients with Zollinger-Ellison syndrome, 16 patients with carcinoid tumors, and 16 patients with other types of functioning (n = 6) or nonfunctioning (n = 10) endocrine tumors. 111In-Pentetreotide planar images (head, chest, abdomen, and pelvis) were obtained 4 and 24 h after injection of 10 μg somatostatin analog labeled with 148 ± 17 MBq 111In, and SPECT was performed 24 h after injection. Similar 99mTc-P829 planar images were obtained at 1, 4–6, and 24 h after injection of 50 μg peptide labeled with 991.6 ± 187.59 MBq 99mTc. Abdominal SPECT was performed 4–6 h after injection. Results: 111In-Pentetreotide detected 203 tumoral sites in 39 (91%) of 43 patients, whereas 99mTc-P829 detected 77 sites in 28 (65%) of 43 patients (P < 0.005). In the liver, 129 sites (in 24 patients) were detected by 111In-pentetreotide scintigraphy and 34 sites (in 10 patients) were detected by 99mTc-P829 scintigraphy. Conclusion: In patients with endocrine tumors, the detection rate of 99mTc-P829 scintigraphy was lower than that of 111In-pentetreotide scintigraphy, which appeared to be more sensitive, especially for liver metastases.
Most endocrine tumors express cell-surface somatostatin receptors (1–8). Scintigraphic detection of specific cellular expression is available using an indium-labeled somatostatin analog receptor tracer with a high affinity for binding to a receptor or cell product (9–16). Pentetreotide is an analog of octreotide produced by the synthetic addition of diethylenetriaminepentaacetic acid, which provides 4 COOH groups for the formation of metal-binding complexes. 111In-Pentetreotide (OctreoScan; Mallinckrodt, Inc., St. Louis, MO) is commercially available for imaging. 111In-Somatostatin receptor scintigraphy is a sensitive method for the detection of endocrine tumors and their metastases (17–24). However, the potential clinical advantage of technetium labeling, compared with indium labeling, is related to lower cost, better availability, and faster tumoral visualization (1-d-protocol imaging). In addition, the physical characteristics of technetium are more adapted to the energy detection ability of gamma cameras, with a shorter physical period leading to a higher-dose administration of radiolabeled peptide and better image quality with lower radiation doses. Therefore, the technetium-labeled somatostatin analog (99mTc-P829) was developed (25). P829 is a peptide that contains a bioamino-acid-active sequence mimetic of native somatostatin. 99mTc-P829 was considered to have a biodistribution and receptor binding affinity similar to those of 111In-pentetreotide (26,27). P829 peptide has the molecular formula C65H95N16O12S2 and the structure R-Tyr-(d-Trp)-Lys-Val-R′-(β-Dap)-Lys-Cys-Lys-amide.
The purpose of this study was to prospectively evaluate the ability of scintigraphy using the somatostatin analog labeled with 99mTc (99mTc-P829), compared with 111In-pentetreotide scintigraphy, to detect and localize endocrine tumors. The results of 99mTc-P829 and 111In-pentetreotide scintigraphy were compared, with the latter considered the gold standard scintigraphic imaging procedure for the detection of endocrine tumors and their metastases. This study was part of a multicenter trial comprising 3 French centers (at Lyon, Rennes, and Paris).
MATERIALS AND METHODS
Patients
Forty-three patients (32 men, 11 women; age range, 24–78 y; mean age, 56 ± 8 y) with proven endocrine tumors were prospectively included at 3 French centers. Written consent was obtained from all patients. The study population included 2 patients with a growth hormone-producing pituitary adenoma, 1 patient with Cushing’s syndrome and with a suspected adrenocorticotropic hormone (ACTH)-secreting tumor, and 40 patients with gastroenteropancreatic (GEP) tumors. The final diagnosis of growth hormone-producing pituitary adenoma was obtained by surgery after scintigraphy. In the patient with Cushing’s syndrome and with a suspected ACTH-secreting tumor, no evidence of tumor was found by conventional imaging.
Among the 40 patients with GEP tumors, 30 patients had functioning tumors: 11 patients with Zollinger-Ellison syndrome, based on evidence of the specific biologic syndrome (n = 11) or histopathology findings (n = 9); 16 patients with a histologically confirmed carcinoid tumor; 2 patients with a glucagonoma, and 1 patient with an insulinoma. Ten patients had endocrine tumors that were proven histologically but were classified as nonfunctioning because the patients lacked any specific clinical symptoms related to hormone overproduction markers (e.g., insulin, gastrin with secretin, vasoactive intestinal peptide, glucagon, somatostatin, or serotonin).
Final diagnoses in patients considered to have a GEP tumor or metastases were based on the results of radiology (CT, MRI, arteriography, or endoscopic sonography) or of surgery and histology. In patients with multiple metastases, confirmation of metastatic involvement was not always available for all sites but was available for at least 1 tumoral site. According to radiologic findings or histology, 24 patients had known liver metastases and 4 patients had bone metastases. During the follow-up after scintigraphy, 8 patients underwent surgery (2 patients with pituitary adenomas and 6 patients with GEP tumors). Altogether, histologic confirmation of tumor was obtained (before or after scintigraphy) for 41 of 43 patients. Of the 2 patients without histologic confirmation, 1 had specific biologic evidence of Zollinger-Ellison syndrome but no evidence of tumor and 1 had Cushing’s syndrome with suspicion of, but no evidence of, an ACTH-secreting tumor.
111In-Pentetreotide and 99mTc-P829 scintigraphy were performed on each patient within 3 wk of each other.
111In-Pentetreotide Imaging
A mean dose of 148 ± 17 MBq 111In-pentetreotide (Mallinckrodt Medical, Petten, The Netherlands) containing 10 μg somatostatin analog was administered immediately after the specific radiochemical purity had been checked by chromatography (mean purity, 96.90% ± 1.85%).
Scintigraphic planar images were acquired using a double-head camera (DST or DST XL; SMV, Brie, France) with a medium-resolution parallel-hole collimator, a 256 × 256 word matrix, and a preset time of 10–15 min. Acquisition was performed using both 111In photopeaks (171 and 245 keV).
Abdominal images were obtained 4 h after injection, in the anterior and posterior views. For 12 patients, 1-h images were obtained to compare 111In-pentetreotide uptake with 99mTc-P829 uptake. At 24 h, the acquisition systematically included anterior and posterior views of the head, chest, abdomen, and pelvis. Additional lateral and oblique views were obtained when necessary. Delayed images of the abdomen were systematically obtained in the anterior and posterior views at 30–48 h after injection. When findings were negative or doubtful, acquisition time was increased from 15 to 20 min.
Abdominal SPECT was performed 24 h after injection on 37 patients. Cerebral SPECT was performed on 2 patients. The SPECT acquisition parameters were a double-indium-peak acquisition, 64 projections over a 360° rotation, 40–60 s per step, and a 64 × 64 matrix. Tomographic slices were obtained using iterative reconstruction (2 iterations, 8 subsets) with Hanning postfilter reconstruction.
99mTc-P829 Imaging
Vials of somatostatin analog containing 50 μg P829 (Diatide, Londonderry, NH) were labeled with 1,061.9 ± 138.1 MBq 99mTc in a volume of 1 mL. The 99mTc-P829 was visually inspected for clarity and particles, and radiochemical purity (labeling yield) was then tested using instant thin-layer chromatography. The mean radiochemical purity was 95.06% ± 2.57%.
Safety was assessed by following up clinical signs and symptoms, with monitoring of vital signs and adverse events after intravenous administration of 99mTc-P829.
Scintigraphic planar images were acquired after injection of 991.60 ± 187.59 MBq 99mTc-P829, using the same double-head camera with a high-resolution parallel-hole collimator, a 256 × 256 word matrix, and a preset time of at least 10 min. Acquisition was performed using a 140-keV technetium photopeak (with a 20% window). Anterior and posterior views of the head, chest, upper abdomen, and lower abdomen were obtained at 1, 4–6, and 24 h after injection.
Abdominal SPECT was performed on the same 37 patients at 4–6 h after injection. Cerebral SPECT was performed on the same 2 patients. The SPECT acquisition parameters were a 140-keV 99mTc peak acquisition, 64 projections over a 360° rotation, 40–60 s per step, and a 64 × 64 matrix. Tomographic slices were obtained using iterative reconstruction (2 iterations, 8 subsets) with Hanning postfilter reconstruction.
Image Analysis
Scintigraphic images were visually analyzed, separately and independently for each scintigraphic method, by 2 independent observers who were unaware of the clinical presentation. A consensus reading was obtained in cases of interobserver disagreement. Images were evaluated for the presence or absence of abnormal uptake in each of the areas, for a total of 6 anatomic regions: head and neck, chest, upper abdomen (excluding the liver), liver, lower abdomen, and bone. For both scans, especially of the liver, if fewer than 10 hot spots were found, the sites were counted. If more than 10 hot spots sites were found, especially in the liver, metastases were considered multiple and counted as 10 lesions.
Quantitative analysis was used to compare 111In-pentetreotide with 99mTc-P829 for uptake and image quality. For the comparison of physiologic tracer accumulation, scintigraphic images were acquired with both tracers at 1, 4, and 24 h after injection (n = 12). Lung-to-background (muscle) uptake ratios and liver-to-background (muscle) ratios were determined at 1, 4, and 24 h for 111In-pentetreotide images and 99mTc-P829 images using the same regions of interest over lung, liver, and muscle for the 2 tracers. Also, tumor-to-background uptake ratios were determined both for 24-h 111In-pentetreotide images and for 4-h (and 1-h, when obtained) 99mTc-P829 images using regions of interest over the lesions and the surrounding homolateral normal-uptake side. The mean counts over the lesions and background in the same regions were calculated for 111In-pentetreotide and 99mTc-P829 scintigraphic images.
Statistical Analysis
The McNemar test was used to compare tumoral site detection by the 2 techniques, and the paired t test was used to compare uptake ratios (P < 0.05 was considered statistically significant).
RESULTS
No adverse reactions were observed in any patients.
Tracer Accumulation
With 111In-pentetreotide scintigraphy, physiologic uptake was seen in the pituitary, thyroid gland, liver, spleen, and kidneys. Bladder activity and bowel contamination were seen. With 99mTc-P829 scintigraphy, physiologic uptake was seen in the pituitary, thyroid gland, lung, bone marrow, liver, spleen, and kidneys. Bladder activity and bowel contamination were seen and appeared more marked than on 111In-pentetreotide images.
Compared with 111In-pentetreotide scintigraphy, 99mTc-P829 scintigraphy showed a higher nonspecific uptake in the thorax (lung and bone marrow) (at 4 h, 3.54 ± 2.2 vs. 4.44 ± 1.83, P < 0.0004; at 24 h, 3.21 ± 0.56 vs. 3.75 ± 0.82, P < 0.03). In the liver, uptake was also higher with 99mTc-P829 scintigraphy at 4 h and was similar at 24 h (at 4 h, 7.82 ± 2.31 vs. 3.49 ± 0.66, P < 0.0006; at 24 h, 8.7 ± 2.45 vs. 7.3 ± 2.12, not statistically significant).
Tumoral Site Detection
The results are summarized in Table 1. 111In-Pentetreotide scintigraphy showed abnormal findings in 39 (91%) of 43 patients and detected 203 tumoral sites. 99mTc-P829 scintigraphy showed abnormal findings in 28 (65%) of 43 patients and detected 77 tumoral sites (P < 0.005) (Figs. 1 and 2).
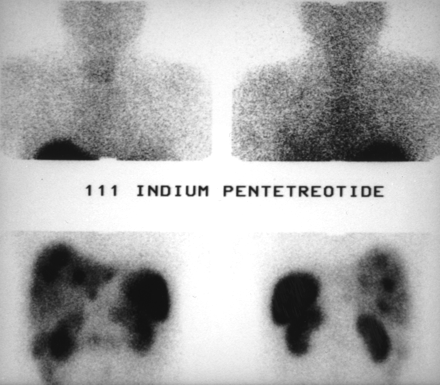
Patient with nonfunctioning endocrine tumor. 111In-pentetreotide scintigraphy shows celiac tumors with liver metastases.
Same patient as in Figure 1. Celiac tumoral and liver metastases are not shown by 99mTc-P829 scintigraphy.
Number of Tumor Sites Revealed by 111In-Pentetreotide and 99mTc-P829
Focusing on liver metastases, 129 sites (24 patients) were detected by 111In-pentetreotide scintigraphy and 34 sites (10 patients) were detected by 99mTc-P829 scintigraphy (Figs. 3 and 4). All liver metastases detected with 99mTc-P829 scintigraphy were also and better visualized with 111In-pentetreotide scintigraphy.
Patient with Zollinger-Ellison syndrome. 111In-pentetreotide scintigraphy shows multiple celiac tumors with liver and lung metastases.
Same patient as in Figure 3. 99mTc-P829 scintigraphy shows lung metastases (smaller than those shown by 111In-pentetreotide scintigraphy). Celiac tumors are not detected. Liver metastases are not clearly seen.
One hundred nine tumoral sites were detected only by 111In-pentetreotide scintigraphy, whereas 3 tumoral sites (1 orbital and 2 lung and mediastinum) were detected only by 99mTc-P829 scintigraphy.
Among the 4 patients who had no abnormalities detected by 111In-pentetreotide, 1 patient had an elevated serotonin level and a liver metastasis detected by abdominal CT and confirmed by surgery after somatostatin receptor scintigraphy (carcinoid tumor); 1 patient had biologic Zollinger-Ellison syndrome and negative conventional imaging findings; 1 patient had Cushing’s syndrome, suspicion of ACTH-secreting tumor, and negative conventional imaging findings; and 1 patient underwent scintigraphy as a follow-up to previous surgery for resection of primary pancreatic tumor and also had negative conventional imaging and biologic findings.
Image Quality
The ratio of tumoral uptake to normal-tissue uptake was significantly higher for 111In-pentetreotide scintigraphy than for 99mTc-P829 scintigraphy (at 4 h, 5.82 ± 5.99 vs. 3.38 ± 3.97, P = 0.008; at 24 h, 5.03 ± 6.00 vs. 2.27 ± 2.55, P = 0.03). Focusing on liver tumors, the ratio of tumoral uptake to normal-tissue uptake was significantly higher for 111In-pentetreotide scintigraphy than for 99mTc-P829 scintigraphy (at 4 h, 6.69 ± 6.71 vs. 3.56 ± 4.81, P = 0.02; at 24 h, 7.45 ± 8.36 vs. 2.34 ± 3.38, P = 0.04).
DISCUSSION
Somatostatin receptors have been found in a variety of endocrine and nonendocrine tumors, with 5 subtypes of receptors individualized (1–7,15). The specific role of each subtype is not clear. 111In-Pentetreotide, a synthetic analog of somatostatin successfully used for staging and therapeutic management in patients with endocrine tumors, is highly sensitive for tumoral detection (28–33). Uptake of 111In-pentetreotide has been shown to be related to receptor subtypes 2 and 5 (6–9). However, the expense of producing 111In on a cyclotron, the limitation placed on the dose size by the long half-life of 111In, and the need to wait 24–48 h after injection for optimum detection of tumors have led to a search for a technetium-labeled somatostatin analog. 99mTc-P829 is a synthetic somatostatin analog, that is, a peptide containing a bioactive amino acid sequence mimetic to native somatostatin. This radiopharmaceutical is safe, having no adverse clinical events and clearing rapidly from the blood after intravenous injection (25–27,34). Uptake of 99mTc-P829 has been found to be related to receptor subtypes 2 and 5, similar to in vitro uptake of 111In-pentetreotide (26).
In our study, which was a part of a multicenter trial, the results of 111In-pentetreotide scintigraphy were compared with the results of 99mTc-P829 scintigraphy for 43 patients with proven endocrine tumors. Compared with 111In-pentetreotide scintigraphy, 99mTc-P829 scintigraphy showed a significantly higher nonspecific uptake in the lungs and bone marrow. In the liver, uptake was also significantly higher with 99mTc-P829 scintigraphy. In addition, 111In-pentetreotide scintigraphy detected tumoral sites in 91% of patients, whereas 99mTc-P829 scintigraphy detected tumoral sites in 65%. Despite the higher octreotide dose used for the 99mTc-P829 (50 μg) than for 111In-pentetreotide (10 μg), lesion contrast was higher for 111In-pentetreotide than for 99mTc-P829, as reflected by the tumoral uptake ratio. The significant difference found between the tracers suggests that, compared with 111In-pentetreotide, 99mTc-P829 is less adapted to the detection of endocrine tumors.
These findings are probably related to a different in vivo biodistribution for the 2 tracers and, in part, explain the lower tumoral uptake ratio with 99mTc-P829. Another explanation may be that the in vivo receptor binding affinity of 99mTc-P829 is different from that of 111In-pentetreotide.
The lower sensitivity of 99mTc-P829 scintigraphy cannot be related to the labeling procedure. Forty-five patients at 3 different French centers were included in this series; the labeling instructions were carefully followed in all cases, and the radiochemical purity was always found to be at least 90%. However, for lung cancer, Blum et al. (34) reported 99mTc-P829 scintigraphy to have a high sensitivity and a high specificity (respectively, 93% and 88%) in the detection of non-small cell cancer in patients with solitary pulmonary nodules. Malignancy in radiologically indeterminate solitary pulmonary nodules was correctly identified or excluded. The sensitivity and specificity compared favorably with the reported results for 18F-FDG PET imaging. In this study, a comparison with 111In-pentetreotide was not performed. However, in comparison with previously reported results for 111In-pentetreotide in non-small cell cancer (35), these results suggest that 99mTc-P829 is superior and has better imaging characteristics. In this study, both biopsy material and large sections from non-small cell tumors were somatostatin receptor negative. Our results for patients with nonendocrine lung cancer who were included in the multicenter trial reported by Blum et al. were similar and showed a high uptake in lung tumors and metastases.
Another study, of patients with endocrine tumors, found better results for a 99mTc-somatostatin analog using 99mTc-tricine-HYNIC-Tyr3-octreotide than for 111In-pentetreotide (36). However, whole-body clearance and absolute tumoral targeting of this compound need to be optimized, and instead of using tricine as coligand, the investigators used ethylene diamine diacetic acid (37), which has been found to result in complexes with higher stability, lower lipophilicity, lower protein binding, and higher tumor-to-organ ratios (38). The first clinical results reported showed better imaging properties than for 111In-labeled derivatives (39).
CONCLUSION
In a comparison with 99mTc-P829 scintigraphy for detection of endocrine tumors, 111In-pentetreotide clearly remained the most sensitive tracer. The reason for this difference in performance is unclear, considering the similar in vitro biodistributions of the tracers, but may possibly be related to a difference in somatostatin receptor binding affinity in vivo or to a difference in somatostatin receptor binding subtypes.
Footnotes
Received Jul. 12, 2001; revision accepted Mar. 25, 2002.
For correspondence or reprints contact: Rachida Lebtahi, MD, PhD, Service de Médecine Nucléaire, Hôpital Bichat, 46 rue Henri Huchard, 75018, Paris, France.
E-mail: rachida.lebtahi{at}bch.ap-hop-paris.fr