Abstract
Radiolabeled somatostatin analogs are important tools for the in vivo localization and targeted radionuclide therapy of somatostatin receptor–positive tumors. The aim of this study was to compare 3 somatostatin analogs designed for the labeling with 99mTc (where HYNIC is 6-hydrazinopyridine-3-carboxylic acid): 6-hydrazinopyridine-3-carboxylic acid0-octreotide (HYNIC-OC/99mTc-(1)), [HYNIC0,Tyr3]octreotide (HYNIC-TOC/99mTc-(2)), and [HYNIC0,Tyr3,Thr8]octreotide (HYNIC-TATE/99mTc-(3)), using ethylenediamine-N,N′-diacetic acid (EDDA) as a coligand. In addition, we compared the 99mTc-labeled peptides [111In-diethylenetriaminepentaacetic acid0]octreotide ([111In-DTPA]-OC) and [111In-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid0,Tyr3,Thr8]octreotide ([111In-DOTA]-TATE) with regard to the rate of internalization and the biodistribution in AR4-2J (expressing the somatostatin receptor subtype 2) tumor-bearing rats. The main attention was directed toward a potential correlation between the rate of internalization and the tumor or pancreas uptake. Methods: Synthesis was performed on solid phase using a standard Fmoc strategy. Internalization was studied in cell culture (AR4-2J) and biodistribution was studied using a Lewis rat tumor model (AR4-2J). Results: The 5 radiopeptides showed a specific internalization into AR4-2J cells in culture (as shown by blocking experiments). The rate of internalization of the 5 radiopeptides differed significantly according to the following order: 99mTc-(1) ≅ [111In-DTPA]-OC < 99mTc-(2) < 99mTc-(3) ≅ [111In-DOTA]-TATE. All radiopeptides displayed a rapid blood clearance and a fast clearance from all somatostatin receptor–negative tissues predominantly via the kidneys. A receptor-specific uptake of radioactivity was observed for all compounds in somatostatin receptor–positive organs such as the pancreas, the adrenals, and the stomach. After 4 h, the uptake in the AR4-2J tumor was comparable for 99mTc-(2) (3.85 ± 1.0 injected dose per gram tissue (%ID/g)), 99mTc-(3) (3.99 ± 0.58 %ID/g), and [111In-DOTA]-TATE (4.12 ± 0.74 %ID/g) but much lower for [111In-DTPA]-OC (0.99 ± 0.08 %ID/g) and 99mTc-(1) (0.70 ± 0.13 %ID/g). The specificity was determined by blocking experiments using a large excess of [Tyr3]octreotide. 99mTc-(3) displayed the highest tumor-to-kidney ratio (2.5:1), followed by 99mTc(2) (1.9:1) and [111In-DOTA]-TATE (1.7:1). Conclusion: These data show that the 5 radiopeptides are specific radioligands for the somatostatin receptor subtype 2. The rate of internalization correlates with the uptake in the tumor (R2 = 0.75; P = 0.026) and pancreas (R2 = 0.98; P = 7.4·10−5). [Tyr3,Thr8]octreotide derivatives show superiority over the corresponding octreotide and [Tyr3]octreotide derivatives, indicating that [111In-DOTA]-TATE and [99mTc/EDDA/HYNIC]-TATE are suitable candidates for clinical studies.
In the last decade, strong efforts have been undertaken to establish radiopeptides in nuclear oncology (1–3) for targeted tumor diagnosis and therapy.
In vitro autoradiographic studies demonstrate the high density and distribution of highly specific membrane receptors on tumor cells, which represent the molecular basis of this application (4). Somatostatin receptors and their radiolabeled ligands are clearly the prototypes for in vivo localization of neuroendocrine primary tumors and their metastases by scintigraphy (5,6). Other radiolabeled peptides were also studied in vivo for detection of various tumors; vasoactive intestinal peptides were studied for in vivo localization of intestinal and pancreatic adenocarcinoma (7), and technetium labeled gastrin-releasing peptides (GRP) were studied for the localization of GRP receptor-expressing malignancies such as breast and prostate carcinoma (8).
Today, [111In-diethylenetriaminepentaacetic acid0]octreotide ([111In-DTPA0]octreotide/OctreoScan; Mallinckrodt Inc.) is routinely used in nuclear medicine for the localization and staging of somatostatin receptor–positive tumors, but its use is somewhat restricted by the high cost of the cyclotron-produced 111In and its suboptimal nuclear characteristics such as the medium-energy photons (171 keV, 245 keV) (9). Other radioligands based on somatostatin analogs have been developed for different purposes: 86Y (10), 68Ga (11,12), 64Cu (13,14), and 18F (15,16) for PET, whereas [90Y-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid0,Tyr3]octreotide ([90Y-DOTA0, Tyr3]octreotide/[90Y-DOTA]-TOC) (17–19) and [177Lu-DOTA0,Tyr3,Thr8]octreotide ([177Lu-DOTA]-TATE) (20,21) were successfully introduced into peptide receptor–mediated radionuclide therapy. Despite the success of 111In-labeled octreotide analogs as imaging agents, somatostatin analogs incorporating the radionuclide 99mTc (half-life = 6.02 h; monoenergetic radiation of 140 keV) would be more desirable. The rapid blood clearance and the fast tumor accumulation of small peptides allow for the use of shorter-lived radionuclides such as 99mTc. Moreover, 99mTc is the most cost-effective radionuclide commonly used in nuclear medicine and is widely available. The bifunctional chelator approach was applied, including bifunctional versions of propylenediaminedioxime (22), acyclic tetraamine (9), and triamidomonothiol (23), to label somatostatin analogs with 99mTc. Recently, a synthetic peptide, selected for high somatostatin receptor affinity, which can be labeled with 99mTc and 188Re, has been approved for human use. This peptide, named P829 (depreotide, NeoSpect; GE Healthcare: Amersham Health), has been studied in patients with various tumors. While P829 shows high accumulation in somatostatin receptor–positive neuroendocrine tumors, uptake has also been described in other tumors, such as breast cancer and melanoma, which have previously been reported to express somatostatin receptors only moderately (24).
We (25,26) and others (27) have chosen 6-hydrazinopyridine-3-carboxylic acid (HYNIC) as a technetium ligand for the labeling of somatostatin analogs. It shows great promise because of the likely monodentate character that may allow the use of a variety of coligands, leading to quite different biodistributions (28).
In this article, we compare 3 different somatostatin analogs designed for high specific activity labeling with 99mTc: [HYNIC0]octreotide (HYNIC-OC/99mTc-(1)), [HYNIC0,Tyr3]octreotide (HYNIC-TOC/99mTc-(2)), and [HYNIC0,Tyr3,Thr8]octreotide (HYNIC-TATE/99mTc-(3)), with the commercial gold standard of somatostatin receptor imaging, [111In-DTPA0]-octreotide (OctreoScan), and with the new radioligand [111In-DOTA0,Tyr3,Thr8]octreotide ([111In-DOTA]-TATE).
The synthesis of HYNIC-OC was described some time ago but pharmacologic and biodistribution data were not reported (29). Preclinical evaluation of HYNIC-TOC with different coligands has been covered in the literature and a kit formulation can be used for convenient labeling and clinical application (30,31). [99mTc-HYNIC]-TOC, using ethylenediamine-N,N′-diacetic acid (EDDA) or tricine as coligands to target somatostatin receptor–positive tumors, was successfully studied in an animal model (27) and in humans (32). The substitution of Phe3 by Tyr3 and Thr(ol)8 by Thr8 leading to the peptide called TATE was shown before with other chelators to lead to a higher receptor affinity to the somatostatin receptor subtype 2 (33), to a higher rate of internalization, and, eventually, to a higher tumor uptake (34).
In this study we compare the rate of internalization into AR4-2J tumor cells in culture and the biodistribution and pharmacokinetics in AR4-2J tumor-bearing rats. The main attention was directed toward a potential correlation between the rate of internalization and the tumor uptake as well as the pancreas uptake.
MATERIALS AND METHODS
Reagents and Instrumentation
All chemicals were obtained from commercial sources and used without further purification. H-Thr(tBu)-ol-(2-chlorotrityl)-resin was obtained from Advanced ChemTech, and tritylchloride polystyrene resin was purchased from PepChem. HYNIC-Boc (6-Boc-hydrazinopyridine-3-carboxylic acid) was synthesized according to Abrams et al. (35); DOTA(tBu)3 (1,4,7,10-tetraazacyclododecane-1,4,7-tris(acetic acid-t-butyl ester)-10-acetic acid) was synthesized according to Heppeler et al. (36). The cell culture medium was Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, amino acids, vitamins, and penicillin/streptomycin from Gibco BRL. For sterility filtration, 20-μm Millex-GS filters from Millipore were used. 99mTc, available from a 99Mo/99mTc generator, and 111In were purchased from Mallinckrodt Medical Inc.
Solid-phase peptide synthesis was performed on a semiautomatic peptide synthesizer from Rink Combichem Technologies. Analytic reversed-phase high-performance liquid chromatography (RP-HPLC) was performed on a Hewlett Packard 1050 HPLC system equipped with a multiwavelength detector and a flow-through Berthold LB 506 Cl γ-detector. Preparative HPLC was done on a Bischof HPLC system with HPLC pumps 2250 and a λ-1010 UV detector. CC250/4 Nucleosil 120-3 C18 columns from Macherey-Nagel and a Symmetry 5-μm C18 4.6 × 250 column from Waters were used for analytic HPLC, and a VP250/21 Nucleosil 100-5 C18 column was used for preparative HPLC. The gradient systems consisted of 0.1% trifluoroacetic acid (TFA)/water (solvent A), acetonitrile (solvent B), and 20 mmol/L ammonium acetate buffer, pH 7.0 (solvent C). For analytic HPLC, gradient I was used: 0 min 95% A (5% B), 30 min 55% A (45% B), 32 min 0% A (100% B), 34 min 0% A (100% B), 37 min 95% (5% B), flow: 0.75 mL/min, λ = 214 nm; and gradient II: 0 min 95% C (5% B), 50 min 70% C (30% B), 55 min 0% C (100% B), 57 min 0% C (100% B), 60 min 95% C (5% B), flow: 1.0 mL/min, λ = 214 nm. Electrospray ionization mass spectroscopy (ESI-MS) was performed on a Waters ZMD (Micromass) with a HP1100 Quaternary LC pump. Matrix-assisted laser desorption ionization mass spectroscopy (MALDI-MS) measurements were done on a Voyager sSTR equipped with a Nd:YAG laser (355 nm) (Applied BioSystems). Quantitative γ-counting was performed on a COBRA II, D 5003 γ-system well counter (Canberra Packard). Scintigraphy was performed using a γ-camera with a low-energy, high-resolution, parallel-hole collimator (Diacam). Each image was acquired for 15 min.
Synthesis
The peptide-chelator conjugates were synthesized by standard Fmoc solid-phase synthesis on chlorotrityl resin (substitution: 0.95 mmol/g) for TATE derivatives and O-t-butylthreoninol-2-chlorotrityl resin (substitution: 0.66 mmol/g) for OC and TOC derivatives on a semiautomatic peptide synthesizer. Fmoc was removed by adding 20% piperidine/N,N-dimethylformamide (DMF), coupling reagents were 2.5 eq N-hydroxybenzotriazole (HOBt), 2.5 eq diisopropylcarbodiimide (DIC), and 3 eq N-ethyldiisopropylamine (DIPEA) as base, and standard reaction time was 90 min.
The coupling of HYNIC was done in solution; the fully protected peptides were cleaved from the resin with 0.1% TFA (2-chlorotrityl resin) or 20% acetic acid (chlorotrityl resin), 2 eq HYNIC-Boc were coupled with 2.5 eq O-(7-azabenzotriazol-1-yl)-1,1,3,3,tetramethyluronium hexafluorophosphate (HATU) to the N-terminus of the peptides. Oxidative cyclization using 10 eq iodine, deprotection, and purification by preparative HPLC led to HYNIC-OC, HYNIC-TOC, and HYNIC-TATE (Fig. 1).
Structures of radiopeptides studied.
Complete synthesis of HYNIC-TATE was also performed on solid phase. After assembling the fully protected peptide on the resin, HYNIC-Boc was coupled with HATU to the N-terminus of the peptide followed by cyclization using 1.2 eq thallium(III) trifluoroacetate. After cleavage from the resin and deprotection, the compound was purified by preparative HPLC.
The pro-chelators, DOTA(tBu)3 or DTPA(tBu)3 (2 eq), were coupled with 2.5 eq HATU to the N-terminus of the peptides. Cleavage of the fully protected conjugates from the resin, oxidative cyclization using iodine, deprotection, and purification by preparative HPLC led to compounds DTPA-OC and DOTA-TATE (Fig. 1).
All compounds were lyophilized after purification and characterized by HPLC, ESI-MS, or MALDI-MS. All peptide-chelator conjugates had a purity of >93% confirmed by HPLC (gradient I) (Table 1).
Analytic Data for Each Radiopeptide
Kit Formulation and Radiolabeling with 99mTcO4−
For the labeling of the HYNIC derivatives with 99mTcO4−, a 2-vial kit formulation was used. For HYNIC-TATE, we developed additionally a single-step kit.
One milliliter of a solution containing 15 mg (84 μmol) tricine, 20 μg (16.9 nmol) HYNIC-TATE (HYNIC-TOC, HYNIC-OC), and 40 μg SnCl2 (10 μL of 22.2 mmol/L SnCl2·2 H2O in 0.1 mol/L HCl) was filtrated into a glass vial strictly under air protection. One-half milliliter of a solution containing 5 mg EDDA (pH was adjusted to 7 with 1 mol/L NaOH) was also filtrated into a second glass vial. The glass vials were immediately frozen in liquid nitrogen, lyophilized, and closed afterward under vacuum. For the HYNIC-TATE monovial kit, all compounds were added to the same vial.
For labeling the 2-vial kits, the EDDA vial was reconstituted with 0.5 mL saline and added to the HYNIC-peptide vial. For the labeling of the monovial kit, first 0.5 mL saline was added, and the mixtures were allowed to preincubate for 15 min. Then, 1–3 GBq 99mTcO4− in about 0.5 mL saline were added to the HYNIC-vials and incubated for 10 min at 95°C. After cooling down to room temperature, the reaction mixture was analyzed by HPLC (gradient I) and instant thin-layer chromatography (silica gel 60 with pyridine/acetic acid/water (5:3:1.5) as eluents).
Radiolabeling of DTPA-OC with 111InCl3
An aliquot of 10 μg DTPA-OC was dissolved in 150 μL ammonium acetate buffer (0.25 mol/L, pH 5.5). After adding 222 MBq 111InCl3, the solution was incubated at room temperature for 30 min and then subjected to quality control by HPLC (gradient I).
Radiolabeling of DOTA-TATE with 111In/natInCl3
An aliquot of 10 μg (in 20 μL H2O) DOTA-TATE was dissolved in 150 μL ammonium acetate buffer (0.25 mol/L, pH 5.5). After adding 111 MBq 111InCl3, the solution was heated at 95°C for 30 min. For the internalization experiment, a 3-fold excess of natInCl3 was added additionally and heated again at 95°C for 30 min. Subsequently, [111In-DOTA]-TATE was purified on a Sep-Pak C18 cartridge by eluting the excess of natInCl3 with sodium acetate buffer (0.4 mol/L, pH 5) and the radioligand with methanol to afford high radiochemical purity (≥99%). The quality control was done by HPLC (gradient I).
Internalization Assay
The internalization experiments were performed as described previously in 6-well plates and the sst2-receptor–positive cell line AR4-2J (37,38). Briefly, 1 million cells per well were washed with internalization medium (DMEM) and allowed to adjust to the medium at 37°C for 1 h.
Afterward, approximately 40 kBq (2.5 pmol) 111In/natIn-labeled peptides in a final peptide concentration of 1.67 nmol/L were added to the medium and the cells were incubated at 37°C in 5% CO2. For the 99mTc-labeled compounds, about 150 kBq (2.5 pmol total peptide mass per well) in a final peptide concentration of 1.67 nmol/L were added to the medium and incubated for different periods of time. To determine nonspecific internalization, an excess of octreotide (150 μL of a 1 μmol/L solution) was added. The internalization was stopped at appropriate time points (30 min, 1 h, 2 h, 4 h) by removal of the medium. The cells were washed twice with ice-cold phosphate-buffered saline (pH 7.2) and then washed twice with cold glycine buffer (0.05 mol/L glycine solution, pH adjusted to 2.8 with 1N HCl) for 5 min at 0°C to distinguish between cell surface–bound (acid releasable) and internalized (acid resistant) radioligand. Finally, cells were treated with 1N NaOH at 37°C for 10 min to detach them from plates. The radioactivity of every fraction was measured on a γ-counter and expressed as the percentage of applied activity normalized to 1 million cells.
Animal Studies
For biodistribution studies, 107 AR4-2J rat pancreatic tumor cells were implanted subcutaneously into 6-wk-old male Lewis rats (Harlan). Fourteen days after inoculation of the tumors, 20 MBq (0.35 nmol) 99mTc-(1) diluted in saline (supplemented with 0.1% bovine serum albumin [BSA], pH 7.4; total injected volume = 150 μL; total peptide mass = 0.5 μg) were injected in the vena saphena magna after incision of the femoral skin under inhalation anesthesia (3 vol% isoflurane with 0.6 L O2/min).
In a cocktail experiment, 2 radioligands with different radioisotopes (99mTc, 140 keV; 111In, 171 and 245 keV) were coinjected: 10 MBq (0.175 nmol) 99mTc-(2) with 5 MBq (0.175 nmol) [111In-DTPA]-OC and 10 MBq (0.175 nmol) 99mTc-(3) with 5 MBq (0.175 nmol) [111In-DOTA]-TATE. The total amount of peptide was 0.35 nmol (0.5 μg) in a total volume of 150 μL saline (supplemented with 0.1% BSA, pH 7.4). For determination of nonspecific uptake in tumor or receptor-positive organs, a group of 4 animals was coinjected with 100 μg of [Tyr3]octreotide in 50 μL saline. After 1 and 4 h, the rats (in groups of 3 or 4 animals) were sacrificed, organs of interest were collected, rinsed of excess blood, plotted dry, and weighed, and radioactivity was measured in a γ-counter. The percentage injected dose per gram (%ID/g) was calculated for each tissue. All animal experiments were performed in compliance with the Swiss regulation for animal treatment (Bundesamt für Veterinärwesen, approval no. 789).
Imaging
The rats were injected 20 MBq (0.35 nmol) 99mTc-(3) diluted in saline (supplemented with 0.1% BSA, pH 7.4; total injected volume = 150 μL; total peptide mass = 0.5 μg) in the vena saphena magna after incision of the femoral skin under inhalation anesthesia (3 vol% isoflurane with 0.6 L O2/min). Under isoflurane inhalation anesthesia, the rats were placed on the collimator of a γ-camera. Images were taken after 1 and 4 h following injection of 2 tumor-bearing rats: one was injected with 99mTc-(3) and the other was injected with 99mTc-(3) and, additionally, 100 μg [Tyr3]octreotide as the blocking agent. The acquisition time of each scintigram was 15 min.
Statistical Methods
The calculations of means and SDs for internalization and biodistribution were performed on Microsoft Excel. Correlations between the rate of internalization and tumor or pancreas uptake were tested using linear regression analysis on Microsoft Excel. Graphs and curve fitting was performed on Microcal Origin. ANOVA and Student t test calculations were performed on StatSoft, Inc. STATISTICA, version 6. P values ≤ 0.05 were considered significant.
RESULTS
Synthesis and Radiolabeling
After standard solid-phase synthesis of the octapeptides, the chelators (DOTA, DTPA, and HYNIC) were coupled to the N-terminus of the resin-bound peptides according to Figure 1. The conjugates then were cleaved from the resin, cyclized, and deprotected.
HYNIC-TATE was synthesized using 2 different strategies: In method 1, the cyclization with iodine and the coupling of HYNIC-Boc were done in solution after cleavage from the resin in an overall yield of 14.0%. In method 2, the cyclization using thallium-III-trifluoroacetate and the coupling of the chelator were done on solid phase followed by cleavage from the resin and deprotection in an overall yield of 11.6%.
The composition and structural identity of all compounds—HYNIC-OC, HYNIC-TOC, HYNIC-TATE, DTPA-OC, and DOTA-TATE—were verified by analytic HPLC, ESI-MS, or MALDI-MS (Table 1). The purity was >93%, as confirmed by HPLC methods. The labeling yield of [99mTc/EDDA/HYNIC]-TATE, independent of the kit formulations used, was >95% at a specific activity of 60 GBq/μmol. The HPLC elution times (gradient I) were 21.9 min for 99mTc-(1), 21.6 min for 99mTc-(2), and 21.8 min for 99mTc-(3), indicating that the lipophilicity or hydrophilicity is determined by the 99mTc-EDDA moiety (Fig. 2).
Potential structure of [99mTc/EDDA/HYNIC]-TATE complex.
The labeling yield of [111In-DTPA]-OC was >99% at a specific activity of 44.2 GBq/μmol. A typical HPLC chromatogram of 99mTc-(3) is shown in Figure 3A. The HPLC elution time of [111In-DTPA]-OC (gradient I) was 22.3 min; using a longer gradient (gradient II) with 0.1% TFA/water or 20 mmol/L ammonium acetate buffer as solvent, the HPLC chromatogram showed 2 peaks (Fig. 3B). The 2 peaks were collected separately and analyzed using MALDI-MS, showing identical masses.
(A) RP-HPLC analysis of [99mTc/EDDA/HYNIC]-TATE (99mTc-(3)) (gradient I). (B) RP-HPLC analysis of [111In-DTPA]-OC (gradient II). mAu = milliabsorbance units.
The labeling yield of [111In-DOTA]-TATE was >99% at a specific activity of 15.9 GBq/μmol. The HPLC elution time is 23.4 min (gradient I).
Internalization Assays
The AR4-2J rat pancreatic tumor cell line is known to express sstr2 receptors in vivo and in vitro (39). The results of the internalization of the 5 radioligands, 99mTc-(1), 99mTc-(2), 99mTc-(3), [111In-DTPA]-OC, and [111In-DOTA]-TATE, are shown in Table 2 along with [111In-DOTA]-TOC.
Comparison of Rate of Internalization of 99mTc- and 111In-Labeled Peptides in AR4-2J Tumor Cells
The compounds labeled with 99mTc showed a significant difference in their rate of receptor-specific internalization after 4 h according to the following order: 99mTc-(1) (2.4% ± 0.8% of added activity per 106 cells) < 99mTc-(2) (9.9% ± 0.6%; P < 10−5) < 99mTc-(3) (18.8% ± 2.7%; P < 10−4). For the 111In-labeled compounds, the internalization was [111In-DTPA]-OC (3.0% ± 0.4%) and [111In-DOTA]-TATE (21.0% ± 2.3%; P < 10−5). The internalization was strongly reduced in the presence of 0.1 μmol/L octreotide (data not shown). In fact, nonspecific internalization was <1.5% after 4 h, and the surface-bound peptide (acid removable) was <2.0% of the added activity after 4 h.
Animal Biodistribution Studies
The results of biodistribution in AR4-2J tumor-bearing Lewis rats of 99mTc-(1), 99mTc-(2), 99mTc-(3), [111In-DTPA]-OC, and [111In-DOTA]-TATE are summarized in Table 3. All radiopeptides displayed a rapid blood clearance with <0.1 %ID/g remaining in blood after 4 h. There was also fast clearance from all sstr-negative tissues, predominantly via the kidney and the urinary system. All compounds showed a high uptake of radioactivity in receptor-positive organs, such as the pancreas, adrenals, and stomach. This uptake was shown to be receptor specific, given the significant reduction of the uptake in the group of animals receiving an excess of unlabeled [Tyr3]octreotide (100 μg). The reduction was >93% in the tumor, >94% in the pancreas, >86% in the stomach, and >84% in the adrenals. The uptake in nontarget tissues was not influenced by the blocking dose.
Biodistribution Data (%)ID/g Tissue ± SD; Each Value is Average of 3 or 4 Animals
The uptake in the AR4-2J rat pancreatic tumor after 4 h was comparable for 99mTc-(2) (3.85% ± 1.00%), 99mTc-(3) (3.99% ± 0.58%), and [111In-DOTA]-TATE (4.12% ± 0.74%). 99mTc-(1) (0.70% ± 0.13%) and [111In-DTPA]-OC (0.99% ± 0.08%) showed a lower, but still specific, uptake than the other compounds. Comparing the tumor-to-kidney ratios, 99mTc-(3) had the highest ratio, 2.5:1, followed by 99mTc-(2) (1.9:1), and [111In-DOTA]-TATE (1.7:1). 99mTc-(1) (0.3:1) and [111In-DTPA]-OC (0.6:1) showed the lowest tumor-to-kidney ratio. Some important tumor-to-normal tissue and receptor-positive organ-to-normal tissue ratios are also given in Table 3.
Imaging
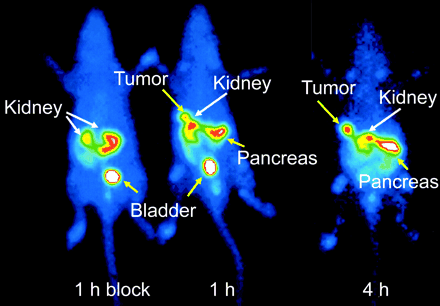
Scintigraphy of a rat showed a specific high uptake in the pancreas and the implanted tumor after 1 h. The administration of excess unlabeled peptide (100 μg [Tyr3]octreotide) to a control animal reduced the uptake in receptor-positive organs and the tumor (Fig. 4). After 4 h, because of background clearance, the tumor-to-normal organ ratio improved in the nonblocked animal.
Scintigraphy of an AR4-2J tumor-bearing rat injected with 20 MBq [99mTc/EDDA/HYNIC]-TATE (right rat). Blocking studies by coinjection of 100 μg [Tyr3]octreotide (left rat).
DISCUSSION
Somatostatin receptor scintigraphy with [111In-DTPA0]octreotide (OctreoScan) has become the gold standard for the localization, staging, and management of neuroendocrine tumors. 111In is not an ideal radionuclide considering its limited availability, suboptimal imaging properties, elevated radiation burden, and the high price. Therefore, in the past 10 y, several 99mTc-labeled somatostatin analogs have been developed that can be easily labeled at high specific activities—among them, P829, the first registered 99mTc-based radiopeptide (NeoSpect). It shows good results in the identification of solitary nodules in patients with non–small cell lung cancer. In patients with endocrine tumors, the detection rate using P829 scintigraphy was lower than that of OctreoScan scintigraphy, which appeared to be more sensitive, especially for liver metastases, because of the high liver uptake of P829 (40).
In this study, we compared a series of 99mTc-labeled octreotide derivatives (HYNIC-OC, HYNIC-TOC, and HYNIC-TATE) with [111In-DOTA]-TATE and with the commercially available [111In-DTPA]-OC. Internalization was studied with emphasis on a potential correlation between the rate of internalization, tumor uptake, and uptake in the somatostatin receptor–positive organs, such as the pancreas.
The 99mTc labeling was straightforward for all HYNIC derivatives and for both kit formulations. Starting with a 2-vial kit with high radiochemical yields (>99%) and specific activities of about 60 GBq/μmol, the monovial kit gave somewhat lower radiochemical yields (>95%) at the same specific activity. The HPLC chromatogram of [111In-DTPA]-OC showed 2 peaks of the 2 major isomers of monoamide-modified InIII-DTPA. MALDI-MS analysis confirmed the formation of 2 isomers. This is expected, as the central nitrogen of DTPA-monoamide is prochiral and becomes chiral on metal complex formation.
The 5 radiopeptides showed a receptor-specific internalization into AR4-2J rat pancreatic tumor cells. There is a distinct tendency with regard to the rate of internalization: Octreotide-conjugates ([111In-DTPA]-OC, 99mTc-(1)) internalize at the same rate (P values between 0.12 and 0.37 at 4 different time points) but much slower than [Tyr3]octreotide-conjugates ([111In-DOTA]-TOC, 99mTc-(2); P values = 0.16 and 0.83 at 2 and 4 time points, respectively) and these internalize significantly slower than the [Tyr3,Thr8]octreotide-conjugates ([111In-DOTA]-TATE, 99mTc-(3); P values between 0.06 and 0.79 at 4 time points), which again internalize at the same rate. Between the groups, the Kruskal–Wallis ANOVA test gave significant differences at all time points (P values < 3·10−4). A very similar trend was also found by Lewis et al. studying [64Cu-TETA]-conjugated octreotide, [Tyr3]octreotide, and [Tyr3,Thr8]octreotide in the same cell line (34).
The distinct difference in the rate of internalization is also clearly reflected in the pharmacokinetics in AR4-2J tumor-bearing rats. The uptake in tumor and somatostatin receptor–expressing pancreas follows the order of the internalization rate (Fig. 5). We found a good correlation between the rate of internalization at 4 h and the uptake in the tumor (R2 = 0.75; P = 0.026) and in the pancreas (R2 = 0.98; P = 7.4·10−5). The lack of significance between the tumor uptake of 99mTc-(2) and 99mTc-(3) (P = 0.404) may be related to a somewhat low blood flow in the AR4-2J tumor, which limits the tumor uptake.
Correlation of rate of internalization (% specific internalized/106 cells) and tumor or pancreas uptake (%ID/g tissue), both at 4 h. Every data point shows % specific internalization ± SD and %ID/g tissue ± SD, respectively. Values for [111In-DOTA]-TOC are from Ginj et al. (37) or are unpublished data. 1 mio = 1 million.
The scintigraphy of the tumor bearing Lewis rats on a γ-camera showed a specific uptake in the pancreas and the tumor after 1 h. All receptor-positive organs and the tumor were blocked by the addition of an excess of [Tyr3]octreotide. At 4 h, because of background clearance, the tumor-to-normal organ ratio improved. Therefore, this radioligand may be used in the clinic successfully and at very early time points.
CONCLUSION
In this study, we compared 3 99mTc-labeled somatostatin analogs and [111In-DOTA]-TATE with the standard [111In-DTPA]-OC by modifying the amino acid position (amino acid positions 3 and 8) of the peptide and, on the other hand, by the change from 111In with its suboptimal nuclear characteristics to 99mTc.
In addition, we studied a potential correlation between in vitro internalization and in vivo tumor uptake and the somatostatin receptor–positive pancreas uptake for 5 somatostatin-based radioligands in the same assays.
The general picture is that radioligands based on [Tyr3,Thr8]octreotide are superior to those based on [Tyr3]octreotide and octreotide, respectively; this holds true for the 99mTc- and 111In-labeled conjugates. The tumor and pancreas uptake correlates with the rate of internalization. There seems to be no significant difference between the corresponding 99mTc- and 111In-labeled peptides. On the basis of these preclinical data, 99mTc-(3) and [111In-DOTA]-TATE may well be superior radioligands for the imaging of somatostatin receptor–positive tumors in the clinic than existing radioligands, and 99mTc-(3) may be considered the first choice because of the well-known advantages of this radionuclide in diagnostic nuclear medicine. Initial clinical studies indicated that the higher hydrophilicity and the higher tumor-to-liver ratio of 99mTc-(3) compared with 99mTc-(2) may consequently lead to an improved sensitivity in the detection of liver metastases.
Acknowledgments
Financial support from the Swiss National Science Foundation (grant 3100AO-100390) is gratefully acknowledged. This work was performed within the European Cooperation in the field of Scientific and Technical Research, COST Action B12: “Radiotracers for the in vivo assessment of biologic functions.”
Footnotes
Received Mar. 7, 2005; revision accepted May 26, 2005.
For correspondence or reprints contact: Helmut R. Mäcke, PhD, Division of Radiological Chemistry, University Hospital Basel, Petersgraben 4, CH-4031 Basel, Switzerland.
E-mail: hmaecke{at}uhbs.ch.