Abstract
Recent studies have clarified that hemodynamically compromised patients are at high risk for subsequent stroke. The acetazolamide test is widely used to detect the patients with hemodynamic compromise due to occlusive carotid artery disease. Previous studies have suggested that patients with impaired reactivity to acetazolamide had an increased oxygen extraction fraction (OEF) on PET. However, the underlying pathophysiology has not been defined in patients with reduced blood flow and preserved reactivity to acetazolamide due to carotid occlusive diseases regardless of a normal appearance on MRI. This study aimed to clarify hemodynamic and metabolic parameters in such patients, using 15O gas and 11C-flumazenil (FMZ) PET. Methods: Our study included 15 patients who had reduced cerebral blood flow (CBF) and preserved cerebrovascular reactivity (CVR) to acetazolamide in the ipsilateral middle cerebral artery territory due to occlusive carotid diseases on N-isopropyl-p-123I-iodoamphetamine (123I-IMP) SPECT. We determined the CBF, cerebral metabolic rate for oxygen (CMRo2), cerebral blood volume (CBV), and OEF using 15O gas PET. The binding potential for 11C-FMZ was also measured in 5 patients. All patients were medically treated and followed-up during a mean period of 2.7 y. Results: 15O gas PET scans revealed that the ipsilateral CBF and CMRo2 were reduced to 80% ± 11% (P < 0.0001) and 78% ± 8% (P < 0.0001) of the contralateral side, respectively. However, there was no significant side-to-side difference in the CBV and OEF. The ipsilateral binding potential for 11C-FMZ was also significantly reduced to 82% ± 2% of the contralateral side (P < 0.05), being very similar to the asymmetry of the CBF and CMRo2. No patients suffered further ischemic stroke in the ipsilateral hemisphere during the follow-up period. Conclusion: Our results strongly suggest that a reduced CBF and a normal CVR characterize oxygen hypometabolism probably due to ischemia-related neuronal loss—namely, incomplete infarction. Such an ischemic lesion is not hemodynamically compromised and is at very low risk for a subsequent ischemic stroke even if the patient is medically treated.
There is increasing evidence that hemodynamically compromised patients with internal carotid artery (ICA) occlusion are at a high risk for subsequent ischemic stroke. Recently, an elevated oxygen extraction fraction (OEF) determined by PET has been accepted as an independent risk factor for subsequent ischemic stroke (1,2).
Alternatively, cerebrovascular reactivity (CVR) to CO2 or acetazolamide has also been used to assess the cerebral perfusion reserve in patients with occlusive carotid diseases because SPECT or unlabeled xenon CT is more widely available than PET (3–11). Recent studies have proven that quantitative measurements of cerebral blood flow (CBF) and CVR can also be a predictor for subsequent ischemic stroke in patients with ICA or middle cerebral artery (MCA) occlusion (6,7), although it is still controversial whether impaired CVR is closely related to OEF elevation (3,12–16). Based on the quantitative analysis of CBF and CVR, all patients can be simply classified into 4 types: the patients with a normal CBF and CVR (type 1), those with a normal CBF and a reduced CVR (type 2), those with a reduced CBF and CVR (type 3), and those with a reduced CBF and a normal CVR (type 4) (4–6). According to previous studies, type 1 patients are considered to have a normal cerebral perfusion pressure (CPP) because of a well-developed collateral circulation. Type 2 patients have moderately reduced CPP. Type 3 patients are believed to have inadequate collaterals to maintain a normal CPP (4–6).
However, to our knowledge, there are no reports in the literature that definitely document the pathophysiology in type 4 patients, who have a reduced CBF and a normal CVR. Previous studies have revealed that extracranial–intracranial arterial bypass had no significant effect on their SPECT parameters (5) and that subsequent ischemic stroke is quite rare when they are treated medically (6). These results strongly suggest that chronic cerebral ischemia has already caused partial tissue damage, leading to a decreased metabolic demand, regardless of a normal appearance on CT or MRI, although metabolic parameters have not precisely analyzed in these patients. Therefore, in this study, we aimed to specify the underlying pathophysiology in type 4 patients who have a reduced CBF and a preserved CVR to acetazolamide. For this purpose, we measured the parameters for oxygen metabolism and for neuronal integrity in type 4 patients with occlusive carotid artery disease using 15O gas and the 11C-labeled benzodiazepine receptor ligand flumazenil (11C-FMZ) PET, respectively.
MATERIALS AND METHODS
This study included a total of 15 patients (12 men, 3 women; mean age, 65.3 y; range, 59–72 y) who were admitted to our hospital between January 1999 and February 2002. All of them met the following criteria: (a) transient ischemic attack or minor completed stroke (Rankin score, 1 or 2) due to ipsilateral occlusive carotid artery disease; (b) severe stenosis (>90%) or occlusion of the ipsilateral ICA or MCA; (c) no infarction or, if any, small infarction on MRI; and (d) decreased CBF and normal CVR to acetazolamide in the ipsilateral MCA territory on SPECT. Digital subtraction angiography showed ICA occlusion in 9 patients, ICA severe stenosis in 3, and MCA severe stenosis in 3 (Table 1). All studies were performed at least 4 wk after the last ischemic episode because the studies in an earlier period might affect the correct interpretation of the data (5).
Characteristics of 15 Patients Included in Study
All patients were medically treated with aspirin (81–100 mg/d) or ticlopidine (200 mg/d) and were followed-up in the outpatient clinic over a mean period of 2.7 y (range, 0.5–4 y).
123I-IMP SPECT Study Protocol
To determine the CBF and CVR to acetazolamide, SPECT data were acquired from 20 to 40 min after the tracer injection, using a triple-head γ-camera (GCA-9300/DI; Toshiba) equipped with low-energy, high-resolution fanbeam collimators. The energy settings were 160-keV peak with 24% width for the main window. The matrix size was 128 × 128 pixels. The images were reconstructed using the filtered backprojection method. The data were preprocessed using a Butterworth filter with a cutoff frequency of 0.10 cycle per pixel and a power factor of 8. Attenuation correction was performed using the method of Chang (17). The attenuation coefficient was set at 0.10 per cm. Attenuation coefficient values were determined by a phantom study. The imaging resolution was about 10-mm full width at half maximum after reconstruction.
Quantitative blood flow was determined by using the 123I-N-isopropyl- p-123I-iodoamphetamine (123I-IMP) injection and single-scan autoradiographic (ARG) technique as described earlier (9,18–20). The 123I-IMP-ARG method is based on the 2-compartment model for tracer kinetics. The method used a standard arterial input calibrated by the radioactivity of a single arterial whole-blood sample, a standard lipophilic fraction of 123I-IMP in whole blood, and a fixed distribution volume of 123I-IMP (41 mL/mL). Previous studies have demonstrated a good correlation between regional CBF (rCBF) at the resting state measured by PET with H215O and that measured by the 123I-IMP-ARG method (9,18–20). Briefly, 222 MBq (6 mCi) 123I-IMP were infused into the antecubital vein at a constant infusion rate for 1 min. At 10 min after the beginning of the 123I-IMP infusion, one arterial blood sample was taken and its whole-blood radioactivity concentration was counted using a well counter that was cross-calibrated to a SPECT scanner. A single SPECT scan was obtained at a midscan time of 30 min after 123I-IMP injection. The duration of the SPECT scan was 20 min. The rCBF was quantitatively measured before and 15 min after intravenous injection of 10-mg/kg acetazolamide on the separate days with an interval of 2–3 d.
To evaluate cerebral hemodynamics, 10-mm-diameter circular regions of interest (ROIs) were symmetrically placed in the ipsilateral and contralateral MCA territories. As described elsewhere (4–6), the CVR to acetazolamide was quantitatively calculated as:
 where CBFrest and CBFACZ = rCBF before and after intravenous injection of acetazolamide, respectively.
where CBFrest and CBFACZ = rCBF before and after intravenous injection of acetazolamide, respectively.
Normal control values (mean ± SD) of CBF (38.1 ± 5.4 mL/min/100 g) and CVR (30.% ± 8.0%) in the MCA territory on 123I-IMP were obtained from 10 healthy volunteers free of cerebrovascular disease. The values were rated as reduced when any value was less than the mean − 2 SDs. Thus, in this study, the patients were diagnosed as having type 4 ischemia when their CBF was <27 mL/min/100 g and their CVR was >14%.
PET Measurements
PET studies were performed using an ECAT EXACT HR+ scanner (Siemens) with in-plane and axial resolutions of 4.5 and 3.71 mm, respectively. The intervals between SPECT and PET measurements were between 3 and 21 d (9.7 ± 5.3 d). The PET scanner provides 63 tomographic images with 2.425-mm intervals by the continuous axial motion of the gantry. The image slices were parallel to the orbitomeatal line. Before emission scanning, a transmission scan using a 68Ge line source was obtained to correct tissue attenuation. Intermittent arterial blood sampling and β-ray monitoring with a scintillator were conducted throughout PET scanning using a catheter implanted in the radial artery to obtain the arterial input function. Pao2, Paco2, hemoglobin, and the blood pH were also measured in the same blood samples. One-minute inhalation of C15O (2 GBq/min) followed by 3-min static scanning and 3 arterial blood samplings were obtained to measure the cerebral blood volume (CBV). After a 15-min inhalation of 15O2 (0.5 GBq/min), a steady-state O2 image was scanned and 3 arterial blood samplings were obtained for 5 min to measure the OEF and the cerebral metabolic rate for oxygen (CMRo2). Finally, to determine the CBF, the steady-state CO2 image was scanned and 3 arterial blood samplings were obtained for 5 min after a 15-min inhalation of C15O2 (0.5 GBq/min).
The following formulas were used to calculate the CBV, CBF, OEF, and CMRo2:
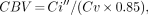



 where Ci″ = CO PET count; Cv = CO blood count; λ = decay constant of 15O (ln2/2.04 min = 0.341); Ca = equilibrium CO2 blood count; Ci = equilibrium CO2 PET count; Ci′ = O2 PET count; Cp′ = O2 plasma count; Cp = equilibrium CO2 plasma count; Ca′ = O2 blood count; OEF′ = corrected OEF with vascular component; X = (CBF + λ)/(CBF/CBV + λ); and 1.39 = maximum binding capacity of oxygen per gram of hemoglobin (mL O2/g hemoglobin).
where Ci″ = CO PET count; Cv = CO blood count; λ = decay constant of 15O (ln2/2.04 min = 0.341); Ca = equilibrium CO2 blood count; Ci = equilibrium CO2 PET count; Ci′ = O2 PET count; Cp′ = O2 plasma count; Cp = equilibrium CO2 plasma count; Ca′ = O2 blood count; OEF′ = corrected OEF with vascular component; X = (CBF + λ)/(CBF/CBV + λ); and 1.39 = maximum binding capacity of oxygen per gram of hemoglobin (mL O2/g hemoglobin).
Normal PET values were obtained from 10 volunteers: CBF, 44 ± 4 mL/min/100 g; CMRo2, 3.3 ± 0.6 mL/min/100 g;, CBV, 3.7 ± 0.7 mL/min; and OEF, 0.43 ± 0.05 (mean ± SD). Each PET parameter was obtained using 10-mm-diameter circular ROIs.
Dynamic FMZ PET was studied in 5 of 15 patients at the same time that 15O gas PET was performed. When the other patients were admitted to our hospital, 11C-FMZ PET was not yet available. The transaxial images of the brain were reconstructed with a voxel size of 1.73 × 1.73 × 2.45 mm3. Photon attenuation was corrected with a 10-min transmission scan and the data were corrected for decay. The injected dose of 11C-FMZ was 370 MBq for each patient. A set of 27 sequential PET frames, of increasing duration, were obtained over a period of 60 min after 11C-FMZ injection according to the following protocol: 40 s × 1 frame, 20 s × 10 frames, 60 s × 4 frames, 180 s × 4 frames, and 300 s × 8 frames. The binding potential (BP) images were calculated pixel by pixel using the reference tissue model (21). The control value in the cerebral cortex was obtained from 11 volunteers and was 4.4 ± 1.5 (mean ± SD) when the pons was used for the reference.
To evaluate cerebral hemodynamic and metabolic parameters, 10-mm-diameter circular ROIs were symmetrically placed in the ipsilateral and contralateral MCA territories.
Data Analysis and Statistical Analysis
To evaluate various parameters obtained from 123I-IMP SPECT, 15O gas PET, and 11C-FMZ PET, the SPECT and PET images were automatically coregistered to axial T1-weighted MR images. The SPECT, PET and MR images were registered using a fully automatic multimodality image registration algorithm (22) on Unix-based workstation (Indigo 2; SGI Inc.).
All data are expressed as mean ± SD. Comparison between the ipsilateral and contralateral sides was evaluated with a paired t test. P < 0.05 was considered to be statistically significant.
RESULTS
SPECT Studies
On SPECT studies, the CBF in the ipsilateral MCA area was 25.6 ± 2.1 mL/100 g/min and was significantly lower than that in the contralateral MCA area (33.3 ± 3.5 mL/100 g/min (P < 0.0001). The ratio of ipsilateral to contralateral CBF was 0.77 ± 0.05. On the other hand, the CVR in the ipsilateral and contralateral MCA areas was 25.1% ± 5.0% and 28.9% ± 7.1%, respectively. The ratio of ipsilateral to contralateral CVR was 0.91 ± 0.25. Thus, there was no significant difference of the CVR between the ipsilateral and contralateral MCA areas (Table 2).
SPECT and 15O Gas PET in 15 Patients Included in Study
15O Gas PET Studies
PET studies also revealed that the CBF in the ipsilateral MCA area was 31.4 ± 4.2 mL/100 g/min and was significantly lower than that in the contralateral MCA area (39.4 ± 3.5 mL/100 g/min, P < 0.0001). The ratio of the ipsilateral CBF to the contralateral CBF was 0.80 ± 0.11, which was almost the same as the results on SPECT studies. The CMRo2 in the ipsilateral MCA area was 2.3 ± 0.4 mL/100 g/min and was significantly lower than that in the contralateral MCA area (3.0 ± 0.3 mL/100g/min, P < 0.0001). The ratio of ipsilateral to contralateral CMRo2 was 0.78 ± 0.08. The side-to-side asymmetry was very comparable between the CBF and CMRo2. On the other hand, there were no significant differences of CBV and of OEF between the ipsilateral and contralateral MCA areas. Thus, the CBV was 3.5 ± 1.2 mL/100 g and 3.6 ± 1.2 mL/100 g in the ipsilateral and contralateral MCA areas, respectively. The ratio of ipsilateral to contralateral CBV was 0.95 ± 0.06. The OEF was within normal limits in the ipsilateral and contralateral MCA areas: 0.40 ± 0.08 and 0.42 ± 0.06, respectively. The ratio of ipsilateral to contralateral OEF was 0.97 ± 0.13 (Table 2).
11C-FMZ PET Studies
To evaluate the neuronal integrity in patients included in this study, 11C-FMZ PET was performed on 5 of 15 patients (patients 2, 10, 11, 12, and 13; Table 1). Their ratio of ipsilateral to contralateral CBF on SPECT was 0.76 ± 0.05. The ratios of CBF, CMRo2, CBV, and OEF were 0.83 ± 0.08 (P < 0.01), 0.80 ± 0.03 (P < 0.01), 1.01 ± 0.11, and 0.94 ± 0.06 in these 5 patients, being similar to the results obtained from the 15 patients (Table 3). The BP for 11C-FMZ was measured in the same ROIs as in 15O gas PET. The BP for 11C-FMZ in the ipsilateral and contralateral MCA area was 3.3 ± 0.4 and 4.1 ± 0.6, respectively. Thus, the BP for 11C-FMZ was significantly lower in the ipsilateral MCA area than that in the contralateral MCA area (P < 0.05). The ratio of the ipsilateral to contralateral BP was 0.82 ± 0.02 (Table 3).
SPECT, 15O Gas, and 11C-FMZ PET in 5 Patients Included in Study
Illustrative Case: Patient 13
A 60-y-old man complained of mild weakness of the right extremities and was admitted to our hospital. Neurologic examinations on admission revealed mild right hemiparesis. He was treated conservatively, and his neurologic symptoms almost disappeared within 24 h after the onset. No cerebral infarction was detected on MRI. Cerebral angiography revealed complete occlusion of the left ICA at the origin. SPECT and PET studies were performed 1 mo after the onset. SPECT studies showed a moderate decrease of the CBF in the left frontoparietal lobe. The CBF in the right and left parietal lobes was 32 and 26 mL/100g/min, respectively. The ratio of the left to right side was 0.81. However, the CVR in the regions was 25% and 23.9%, respectively. Thus, the CVR in both sides was within normal limits.
On 15O gas PET, the CBF in the right and left parietal lobe were 42 and 38 mL/100 g/min, respectively. Likewise, the CMRo2 in the regions was 2.7 and 2.3 mL/100 g/min, respectively. Thus, the CMRo2 ratio of the left to right side was 0.83. The CBV in the regions was 2,5 and 2.8 mL/100 g, respectively. The OEF in the regions was 0.36 and 0.38, respectively. Thus, there were no significant differences in the CBV and OEF between the hemispheres. The 11C-FMZ PET study revealed that the BP in the right and left parietal lobe was 4.4 and 3.7, respectively. The side-to-side asymmetry was 0.83 (Fig. 1).
A 60-y-old man with transient ischemic attack due to left ICA occlusion. T2-weighted MR image shows no cerebral infarction (top left). 123I-IMP SPECT demonstrates mild reduction of CBF and normal reactivity to acetazolamide in left cerebral hemisphere (top right). PET shows mild reduction of CBF and CMRo2 as well as of BP for 11C-FMZ in left cerebral hemisphere (bottom).
Clinical Course
All 15 patients were medically treated with antiplatelet agents. As a result, no patients experienced any further episode of ipsilateral ischemic stroke during their follow-up periods (mean, 2.7 y).
DISCUSSION
The present SPECT/PET study clearly showed that, in patients with a reduced CBF and a preserved CVR to acetazolamide due to occlusive carotid artery diseases, the reduction of blood flow is comparable to that of the oxygen metabolic rate in the ipsilateral MCA areas. Thus, on 15O gas PET, the ipsilateral CBF decreased to 83% of the contralateral value, and the ipsilateral CMRo2 also decreased to 78% of the contralateral value. There were, however, no significant differences of OEF and CBV between hemispheres in these 15 patients. Therefore, the so-called “misery perfusion syndrome” (23) or “stage-2 hemodynamic ischemia” (24) does not exist in these areas, and a decreased blood flow is closely coupled to a reduced oxygen metabolism regardless of the lack of an abnormal intensity lesion on MRI.
Our results mirror previous descriptions—that is, using the 133Xe inhalation method and SPECT, Kuroda et al. (5) reported chronologic changes of the CBF and CVR in 32 patients with ICA occlusion. They divided the patients into 4 types based on quantitative SPECT measurements. Eight of 32 patients (25%) had a reduced rCBF and a normal regional CVR (rCVR) in the ipsilateral MCA area (type 4). Their follow-up blood flow studies revealed no significant changes in the rCBF and rCVR whether or not they underwent bypass surgery. Although some of them showed exacerbation of dementia, a subsequent ischemic stroke was not noted during follow-up periods (5). Furthermore, a recent study by Kuroda et al. (6) also revealed that subsequent ischemic stroke in the ipsilateral MCA area was quite rare during mean follow-up periods of 38 mo and that its annual risk was 2.4% per year in medically treated patients with a reduced CBF and a normal CVR (type 4). Ogasawara et al. have reported a similar result (7).
Acetazolamide is known to increase the CBF by dilating cerebral arterioles and is widely used to assess cerebral vasodilatory capacity. It is believed that critical reduction of cerebral perfusion pressure induces maximal dilation of cerebral arterioles, causing a reduction of CVR to acetazolamide (3–11). A recent study has clarified that an increase in the CBV, as well as an elevation of the OEF, can be a high risk for subsequent ischemic stroke in patients with occlusive carotid artery diseases (25). In the present study, there was no significant abnormality of the CBV in the areas with a reduced CBF and a normal CVR, correlating well with the assumption that these areas are not hemodynamically impaired and are not at high risk for subsequent ischemic stroke (26).
Using 11C-FMZ PET, we also measured the BP for 11C-FMZ in 5 of the 15 patients to clarify the underlying mechanism of the reduced oxygen metabolism in the areas with a reduced CBF and a normal CVR. Because γ-aminobutyric acid receptors are abundant in the cortex and sensitive to ischemic damage, specific ligands to their subunits—the cerebral benzodiazepine receptors—have been used as markers of preserved morphologic integrity (27,28). Sette et al. (28) analyzed the effects of permanent versus transient (3–6 h) MCA occlusion in baboons and identified in vivo subcortical “infarcts” by the typical CT-hypodense lesion. However, they also detected an approximate 20% decrease of benzodiazepine receptor binding in the CT-intact opercular cortex adjacent to the hypodense area. The authors suggested that this borderline-ischemic cortex where blood was supplied by pial collateral vessels had suffered partial or selective neuronal necrosis without loss of glial cells; as a consequence, the gross tissue structure had remained “intact” on CT (28). Subsequently, Heiss et al. (29,30) measured the CBF and 11C-FMZ binding in patients with acute ischemic stroke and concluded that a reduction of 11C-FMZ binding can reliably estimate irreversible damage of the cerebral cortex, predicting whether the tissue can benefit from reperfusion therapy. Using 123I-iomazenil (123I-IMZ), an alternative benzodiazepine receptor ligand for SPECT, a reduction of 123I-IMZ binding is shown to reflect oxidative hypometabolism caused by neuronal damage in hemodynamically impaired areas in patients with cerebrovascular disease (31,32). The present results showed that its BP was significantly decreased in these areas, and its side-to-side asymmetry was 0.82, being very similar to that of the CBF and of CMRo2. Therefore, our study strongly suggests that a selective loss of neural cells occurs in these areas regardless of a normal appearance on MRI, leading to the reduction of metabolic demand for oxygen. Therefore, a reduction of blood flow is most likely secondary to a decline of oxygen metabolism.
In discussing the pathophysiology in the areas with a reduced CBF and a normal CVR, 2 patients reported by Lassen et al. (33) are worthy of note. These patients suffered a cerebral infarct in the basal ganglia. Cerebral angiography revealed ipsilateral MCA occlusion in both patients. A blood flow study showed that a large cortical area surrounding the infarct had a very low CBF, 20–25 mL/100 g/min (i.e., moderate ischemia). Later autopsy showed >50% loss of neurons in the cortical area, where CT scans detected no hypodense lesion (33). Subsequently, Garcia et al. (34) emphasized the importance of selective neuronal necrosis (incomplete infarction) in human stroke as a pathologic entity. They concluded that selective neuronal necrosis is the consequence of transient or permanent ischemia of moderate severity and that it is not visible on either CT or MRI. Therefore, oxygen hypometabolism and decreased neuronal integrity observed in a type 4 area are most likely analogous to incomplete infarction. Moderate and chronic cerebral ischemia due to occlusive carotid artery disease may lead to selective neuronal loss and subsequent downregulation of oxygen metabolism, reestablishing a flow–metabolism balance at a lower level than the normal condition in the area with a reduced CBF and a normal CVR.
Few studies using SPECT have identified a subgroup of patients with a reduced CBF and a normal CVR to acetazolamide (type 4 ischemia) due to occlusive carotid diseases (11,35). To accurately detect these patients using SPECT, it is very important to evaluate both the rCBF and the rCVR quantitatively. As Yonas et al. (36) pointed out, qualitative analysis of the rCBF and rCVR have low sensitivity (61%) and low specificity (75%) for detecting the patients with impaired reactivity to acetazolamide. Thus, qualitative analysis judged that 34 of 94 patients with ICA occlusion had impaired reactivity to acetazolamide. Quantitative analysis, however, revealed that 17 of these 34 patients (50%) were false-positive (36). Very recently, Ogasawara et al. (37) also reported that quantitative, but not qualitative, analysis of the CVR to acetazolamide can be a reliable predictor of the 5-y risk for subsequent stroke in patients with symptomatic major cerebral artery occlusion. These results strongly suggest that quantitative blood flow measurement would be essential to identify type 4 patients with a reduced CBF and a normal CVR because they frequently have a significant CBF difference between the hemispheres on both pre- and postacetazolamide blood flow imaging (36,37). More importantly, it is clinically crucial to categorize type 4 patients accurately. When the patients are diagnosed to have a reduced CBF and a normal CVR on quantitative blood flow measurements, we should remember the present results and determine a therapeutic strategy for them properly. Thus, based on these findings, their ischemic lesions are not in a hemodynamically compromised condition and are at very low risk for subsequent ischemic stroke even if the patients are treated medically.
CONCLUSION
This study strongly indicates that a reduced CBF and a normal CVR to acetazolamide (type 4 ischemia) characterize oxygen hypometabolism due to ischemia-related selective neuronal damage in patients with occlusive carotid diseases. It is critical to categorize type 4 patients by quantitatively measuring the CBF and CVR and to determine a therapeutic strategy, because they require medical treatment, but not bypass surgery.
Acknowledgments
The authors thank Dr. Jyoji Nakagawara, Nakamura Memorial Hospital, for his helpful discussion.
Footnotes
Received Oct. 9, 2003; revision accepted Jan. 14, 2004.
For correspondence or reprints contact: Satoshi Kuroda, MD, PhD, Department of Neurosurgery, Hokkaido University Graduate School of Medicine, North 15 West 7, Kita-ku, Sapporo 060-8638, Japan.
E-mail: skuroda{at}med.hokudai.ac.jp








