Abstract
Recent studies have indicated that bone marrow stromal cells (BMSC) have the potential to improve neurologic function when transplanted into animal models of central nervous system disorders. However, how the transplanted BMSC restore the lost neurologic function is not clear. In the present study, therefore, we aimed to elucidate whether BMSC express the neuron-specific γ-aminobutyric acid (GABA) receptor when transplanted into brain that has been subjected to cerebral infarction. Methods: The BMSC were harvested from green fluorescent protein–transgenic mice and were cultured. The mice were subjected to permanent middle cerebral artery occlusion. The BMSC or vehicle was transplanted into the ipsilateral striatum 7 d after the insult. Using autoradiography and fluorescence immunohistochemistry, we evaluated the binding of 125I-iomazenil and the expression of GABA receptor protein in and around the cerebral infarct 4 wk after transplantation. Results: Binding of 125I-iomazenil was significantly higher in the periinfarct neocortex in the BMSC-transplanted animals than in the vehicle-transplanted animals. Likewise, the number of the GABAA receptor–positive cells was significantly higher in the periinfarct neocortex in the BMSC-transplanted animals than in the vehicle-transplanted animals. A certain subpopulation of the transplanted BMSC expressed a neuron-specific marker, microtubule-associated protein 2, and the marker protein specific for GABAA receptor in the periinfarct area. Conclusion: These findings suggest that BMSC may contribute to neural tissue regeneration through migrating toward the periinfarct area and acquiring the neuron-specific receptor function.
The therapeutic potential of stem cell transplantation has recently been studied in various pathologic conditions of the central nervous system, because injured central nervous system tissue has limited regenerative capacity. Embryonic stem cells, neural stem cells, and bone marrow stromal cells (BMSC) have been considered candidate source cells for transplantation therapy. BMSC may be the most likely source among them, because they can be the harvested from the patients themselves without ethical or immunologic problems (1–3).
Evidence is increasing that these stem cells could extensively migrate into damaged tissue, partially express the neural cell–specific markers, and improve neurologic function when transplanted into brain that has been subjected to cerebral infarction (4,5). Recent studies have shown that these cells can develop electrical functions simulating the neurons when cultured in vitro (6–9) or when transplanted into the brain (10,11). Despite this recent progress, the precise mechanisms underlying these phenomena are still unclear, especially with regard to BMSC. Thus, a considerable gap between histologic findings and functional recovery, similar to the “missing link” between apes and humans, remains to be bridged. However, it is essential to clarify the underlying mechanism before undertaking clinical trials with stem cell–based approaches for patients with cerebral stroke.
In the present study, therefore, we aimed to evaluate whether transplantation of BMSC into the infarcted brain can improve brain-specific function. For this purpose, we measured binding of 125I-iomazenil, a radioactive ligand selective for the central type of benzodiazepine receptor, because the receptor is a binding site of benzodiazepine in the γ-aminobutyric acid (GABA) receptor and is specifically expressed in neurons. Simultaneously, using the fluorescent immunohistochemistry technique, we studied whether transplanted BMSC express the GABA receptor and microtubule-associated protein 2 (MAP2), a specific neuronal marker.
MATERIALS AND METHODS
Isolation of BMSC
All animal experiments were approved by the Animal Studies Ethical Committee of Hokkaido University Graduate School of Medicine. To harvest BMSC from mice, we aseptically dissected the femurs from 4- to 8-wk-old transgenic mice expressing enhanced green fluorescent protein (GFP) (The Jackson Laboratory), as reported previously (12,13). The adherent cells were passed 3 times.
Permanent Middle Cerebral Artery Occlusion
Male BALB/c mice were purchased from CLEA Japan, Inc. Anesthesia was induced by inhalation of 4.0% isoflurane in 70:30 N2O:O2 and was maintained with 1.5%−2.0% isoflurane in 70:30 N2O:O2. Permanent focal cerebral ischemia was induced by direct occlusion of the middle cerebral artery, as described previously (13). Core temperature was maintained between 36.5°C and 37.5°C during the procedures.
Transplantation of BMSC
The BMSC suspension (n = 5) or the culture medium (n = 7) was transplanted into the ipsilateral striatum 7 d after permanent middle cerebral artery occlusion. The animals were anesthetized as before. The following procedures were performed under aseptic conditions on a cell culture bench (VWP-1000; Nihon Ika Co.). The animals were fixed to a stereotactic apparatus (model DKI-900; David Kopf Instruments), and the cranium was exposed through a midline skin incision. A burr hole was made 2 mm to the right of bregma, using a small dental drill. A 10-μL Hamilton syringe was inserted 3 mm into the brain parenchyma from the surface of the dura mater, and 10 μL of cell suspension (2 × 105 cells) were introduced into the striatum over a period of 5 min, using an automatic microinjection pump (model KDS-310; Muromachi Kikai Co.). All animals were treated with 10 mg·kg−1 of cyclosporin A subcutaneously every day for 4 wk after transplantation (12,13).
125I-Iomazenil Autoradiography
After decapitation at 4 wk after transplantation, the brains of the vehicle-transplanted animals (n = 5) and the BMSC-transplanted animals (n = 7) were quickly removed for 125I-iomazenil autoradiography and immunohistochemistry.
The specimens were frozen in Tissue-Tek optimal-cutting-temperature compound (Sakura Finetechnical Co., Ltd.), and 10-μm-thick coronal sections were mounted on poly-l-lysine–coated glass slides to prepare for the subsequent procedures. Frozen sections at the levels of the striatum and hippocampus were immersed in buffer containing 25 mmol/L KPO4 and 150 mmol/L NaCl (pH 7.4) for 15 min at 4°C. Subsequently, they were incubated with 500 pmol/L 125I-iomazenil in the buffer, which was equivalent to a radioactivity of 40.7 MBq/L, for 16 h at 4°C. After the incubation with 125I-iomazenil, the sections were washed with the buffer 3 times for 5 min at 4°C and dried at room temperature. The imaging plates were exposed to the treated sections for 45 min, and the data were read with a BAS 2500 storage phosphor imager (Fuji Photo Film Co., Ltd.). Captured images were analyzed using Image Gauge, version 4.0 (Fuji Photo Film Co.).
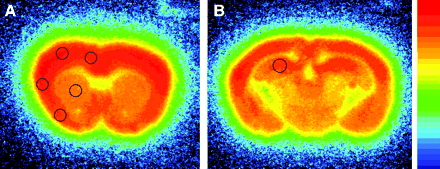
As a preliminary study, we measured binding for 125I-iomazenil in a control mouse. As shown in Figure 1, binding was higher in the neocortex and hippocampus than in the white matter, correlating well with the local density of neurons. The result indicated that the spatial resolution of the images was high enough to measure the binding in each region of interest (ROI). As shown in Figure 1, 6 ROIs were placed—1 each on the neocortex in the anterior cerebral artery territory, the dorsal neocortex adjacent to the ischemic core as the boundary of the infarct, the ischemic core, the ventral neocortex adjacent to the ischemic core, the striatum, and the hippocampus of the ipsilateral hemisphere. Six ROIs were also placed on the equivalent regions of the contralateral hemisphere as controls. The ratio of ipsilateral to contralateral radioactivity was calculated to assess binding of 125I-iomazenil in each ROI.
Representative findings for 125I-iomazenil in vitro autoradiography of control mouse. Two coronal slices through striatum (A) and hippocampus (B) are shown. In this study, ROIs are placed to semiquantitatively evaluate binding for central-type benzodiazepine receptor in neocortex in anterior cerebral artery territory, dorsal neocortex adjacent to cerebral infarct, cerebral infarct, ventral neocortex adjacent to cerebral infarct, striatum, and hippocampus. Same ROIs are also placed in contralateral hemisphere as a control.
Immunohistochemistry
Serially sectioned coronal slices at the levels of the striatum and hippocampus were used for fluorescent immunohistochemistry. Fluorescence immunohistochemistry was performed to measure cell-specific proteins, as reported elsewhere (12,13). Briefly, the sections were treated with the monoclonal antibody against MAP2 (mouse monoclonal, 1:200 dilution; Chemicon) or the polyclonal antibody against the α1 subunit of GABAA receptor (rabbit polyclonal, 1:200 dilution; Santa Cruz Biotechnology, Inc.) labeled with Zenon Alexa Fluor 594 (mouse IgG labeling kit; Molecular Probes Inc.) at 25°C for 1 h. Furthermore, the sections were treated with the monoclonal antibody against GFP (mouse monoclonal, 1:100 dilution; Santa Cruz Biotechnology, Inc.) labeled with Zenon Alexa Fluor 488 (mouse IgG labeling kit; Molecular Probes Inc.) at 25°C for 1 h. The fluorescence emitted was observed through each appropriate filter on a fluorescence microscope (BX51; Olympus) and was digitally photographed using a cooled charge-coupled-device camera (model VB-6000/6010; Keyence Co.).
Statistical Analysis
All data were expressed as mean ± SD. Continuous data were compared between 2 groups by unpaired t testing or among more than 3 groups by 1-factor ANOVA followed by the Fischer test of protected least significant difference. All analyses were performed using StatView, version 5.0 (SAS Institute Inc.). Values of P that were less than 0.05 were considered statistically significant.
RESULTS
All mice that were subjected to permanent middle cerebral artery occlusion and vehicle or BMSC transplantation survived throughout the study and could be used for subsequent analysis.
125I-Iomazenil In Vitro Autoradiography
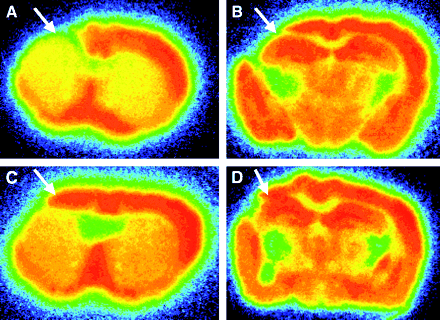
The binding of 125I-iomazenil was measured 4 wk after transplantation of vehicle or BMSC, using in vitro autoradiography. Representative results of vehicle- and BMSC-transplanted animals are shown in Figure 2. Visual evaluations revealed that the binding of 125I-iomazenil was quite low in the ischemic core in both vehicle- and BMSC-transplanted animals, suggesting a marked loss of neurons in the ischemic core. In the vehicle-transplanted animals, the binding of 125I-iomazenil was also low in the dorsal neocortex adjacent to the ischemic core. On the other hand, binding in the area remained subnormal in BMSC-transplanted animals.
Representative findings for 125I-iomazenil in vitro autoradiography of mice subjected to permanent middle cerebral artery occlusion. As shown with arrows, binding for central-type benzodiazepine receptor in dorsal neocortex adjacent to cerebral infarct remained subnormal in BMSC-transplanted mice (C and D), compared with results in vehicle-transplanted mice (A and B).
To semiquantitatively evaluate the binding of 125I-iomazenil, we measured the radioactivity in each ROI as shown in Figure 1. In the anterior cerebral artery territory of the neocortex, the values were almost the same as those in the contralateral hemisphere, and the ipsilateral-to-contralateral ratios were 95.1% ± 6.3% and 97.9% ± 5.1% in vehicle-transplanted animals (n = 5) and BMSC-transplanted animals (n = 7), respectively. The findings were similar in the ventral neocortex adjacent to the ischemic core, the striatum, and the hippocampus. The ipsilateral-to-contralateral ratios in the ischemic core were low, 29.5% ± 8.7% and 32.4% ± 8.2% in vehicle- and BMSC-transplanted animals, respectively. On the other hand, the ipsilateral-to-contralateral ratio in the dorsal neocortex adjacent to the ischemic core was 74.9% ± 11.8% in BMSC-transplanted animals, a value that was significantly higher than the 44.1% ± 18.5% found in vehicle-transplanted animals (P = 0.0053). The results are summarized in Figure 3.
Bar graph showing results of semiquantitative analysis of 125I-iomazenil in vitro autoradiography. Values are ratios of radioactivity in each ROI to radioactivity in corresponding ROI of contralateral hemisphere. As shown in Figure 2, binding for central-type benzodiazepine receptor in dorsal neocortex adjacent to cerebral infarct was significantly higher in BMSC-transplanted mice than in vehicle-transplanted mice (P = 0.0053).
Histologic Analysis
To validate the results in 125I-iomazenil autoradiography, we studied the distribution of GABAA receptor–positive cells in the dorsal neocortex adjacent to the ischemic core, using fluorescence immunohistochemistry. We counted the number of cells immunostaining positively in 2 fields of ×1,000 magnification and calculated the mean values and SDs. As a control, we also calculated the values in the contralateral hemisphere of the vehicle-transplanted animals. The number of GABAA receptor–positive cells was 22.9 ± 4.8 in the contralateral neocortex in vehicle-transplanted animals. The average number of GABAA receptor–positive cells was 15.5 ± 3.2 in BMSC-transplanted animals, a value that was significantly higher than the 11.2 ± 1.8 found in the vehicle-transplanted group (P = 0.0049; Fig. 4). The findings were similar to those of 125I-iomazenil autoradiography.
(A–C) Photomicrographs (original magnification, ×400) of fluorescence immunostaining using antibody against GABAA receptor. Images are of dorsal neocortex adjacent to cerebral infarct of BMSC-transplanted mice (A) or vehicle-transplanted mice (B) or from contralateral neocortex of vehicle-transplanted mice (C). (D) Average numbers of cells positive for GABAA receptor in each area under ×1,000 magnification. Dorsal neocortex adjacent to cerebral infarct had significantly more positive cells in BMSC-transplanted mice than in vehicle-transplanted mice (P = 0.0049). Bars represent SD of each value.
As the next step, double fluorescent immunohistochemistry was performed to evaluate the contribution of the transplanted BMSC to these results. When stained with antibodies against GFP and the GABAA receptor subunit, a certain subpopulation of GFP-positive cells in the dorsal neocortex adjacent to the ischemic core also showed the immunoreactivity of the GABAA receptor subunit (Fig. 5A). Thus, the percentages of cells doubly positive for GFP and GABAA receptor were 42.7% ± 2.6% in total GABAA receptor–positive cells and 72.2% ± 5.4% in total GFP-positive cells. However, a few cells were positive for both GFP and the GABAA receptor subunit in the ipsilateral striatum (Fig. 5B). Likewise, when stained with antibodies against GFP and MAP2, a significant number of GFP-positive cells in the dorsal neocortex adjacent to the ischemic core, but not in the ipsilateral striatum, also expressed MAP2 (Fig. 5C). The findings strongly suggested that a certain subpopulation of the transplanted GFP-positive cells expressed the neuronal phenotypes, GABAA receptor subunit and MAP2, in the periinfarct area after transplantation, because the host neurons do not express GFP.
(A and B) Photomicrographs (original magnification, ×400) of double fluorescence immunostaining using primary antibodies against GFP and GABAA receptor in dorsal neocortex adjacent to cerebral infarct (A) and striatum (B) of BMSC-transplanted mice. Majority of GFP-positive cells express GABAA receptor in dorsal neocortex adjacent to cerebral infarct (arrows) but not in striatum (arrows). (C) Photomicrographs (original magnification, ×400) of double fluorescence immunostaining using primary antibodies against GFP and MAP2 reveal that majority of GFP-positive cells also express neuron-specific protein, MAP2, in dorsal neocortex adjacent to cerebral infarct (arrows) but not in striatum (data not shown).
DISCUSSION
The known decrease in the density of neurons in the periinfarct neocortex is referred to as selective neuronal injury (14). This phenomenon is probably due to a complex pathophysiology including oxygen free radicals, excitotoxicity, repeated depolarization, and immune reactions in the periinfarct area. Therefore, the decreased binding of 125I-iomazenil in the periinfarct cortex of vehicle-transplanted animals most likely represents selective neuronal injury (15). On the other hand, BMSC transplantation significantly improved the binding of 125I-iomazenil in the periinfarct area. Immunohistochemical studies also confirmed that a certain subpopulation of transplanted BMSC migrated toward the periinfarct area and expressed the neuronal phenotypes, including the α1 subunit of GABAA receptor and MAP2. These findings indicate that BMSC transplantation may assist the recovery of receptor function in the periinfarct neocortex. Interestingly, the transplanted cells actively expressed a GABAA receptor subunit and MAP2 in the periinfarct neocortex but not in the striatum. The microenvironment of the transplanted cells in the periinfarct area may have a major impact on their fate.
To our knowledge, this is the first report showing that transplanted BMSC can improve neuron-specific receptor function in brain that has been subjected to cerebral infarction. GABA is known to be an important neurotransmitter in the brain, and the GABAA receptor is the major inhibitory ion channel in the mammalian brain (16). The central type of benzodiazepine receptor is restricted to the nervous tissue and is located between the α1 and γ2 subunits of the GABAA receptor (17,18). The α1 subunit has been described as the main subunit (18). Fluorescence immunohistochemistry against GABAA receptor shows only the existence of the epitope on the α1 subunit of the GABAA receptor. Thus, positive staining against the GABAA receptor subunit does not always indicate receptor function.
Evidence is increasing that transplanted stem cells improve the function of various organs such as the liver and pancreas. Lagasse et al. intravenously injected bone marrow cells into fumarylacetoacetate hydrolase–deficient mice, an animal model of fetal hereditary tyrosinemia type I, and found improvement in their biochemical liver function and life span (19). In addition, Hess et al. transplanted bone marrow–derived cells expressing kit to mice with streptozotocin-induced pancreatic damage and found that the transplanted cells improved insulin production and blood sugar levels by initiating endogenous pancreatic tissue regeneration (20).
Likewise, recent studies have gradually illuminated the mechanism through which cell transplantation regenerates the injured neural tissue and improves its function. Thus, cultured embryonic stem cells and neural stem cells have been reported to become capable of electrically responding to transmitters such as GABA and glutamate and of expressing GABAA receptor (6–8). More interesting, Kim et al. transplanted midbrain-specific dopamine neurons generated from embryonic stem cells to a rat model of Parkinson's disease and found evidence suggesting synaptic contacts between grafted cells and host neurons (11). Benninger et al. applied embryonic stem cell–derived neural precursors to hippocampal slice cultures and reported that the incorporated donor cells expressed α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate and GABAA receptors and received input via host axonal projections on immunohistochemistry and patch-clamp (9). Using patch-clamp technique, Englund et al. demonstrated that neural stem cells transplanted into the hippocampus of postnatal rats could generate action potentials and receive excitatory and inhibitory synaptic inputs from the surrounding cells (10). As mentioned before, however, it is still unclear how the transplanted BMSC contribute to functional recovery in animal models of neurologic disorders (21). In this study, the finding that the transplanted BMSC expressed GABAA receptor and MAP2 in the periinfarct neocortex strongly suggests that the transplanted BMSC may contribute to the improved receptor function around a cerebral infarct.
In most of the previous clinical trials, neurologic outcome has been recognized as the most conclusive evidence that cell transplantation therapy has been beneficial. However, recent studies have shown that both 123I-iomazenil SPECT and 11C-flumazenil PET are reliable modalities to noninvasively evaluate neuronal integrity in the human brain (22–24). Therefore, the present results strongly suggest that, when clinically applied, these techniques may provide important information for assessing the effects of cell transplantation therapy on neurologic function.
CONCLUSION
Using 125I-iomazenil autoradiography and immunohistochemistry techniques, the present study clearly showed that transplanted BMSC have the potential to improve neuronal receptor function in the periinfarct cortex, suggesting that transplanted BMSC have the capacity to improve neuron-specific receptor function in brain that has been subjected to cerebral infarction. The results may help fill in a piece of the “missing link” between histologic findings and functional recovery in animal experiments and may be useful for further stem cell research.
Acknowledgments
This study was supported by Grants-in-Aid 14370424 and 15390426 from the Ministry of Education, Science, and Culture of Japan. The authors sincerely thank Yumiko Shinohe for her technical assistance with cell cultures and histologic analysis.
References
- Received for publication November 3, 2005.
- Accepted for publication December 15, 2005.