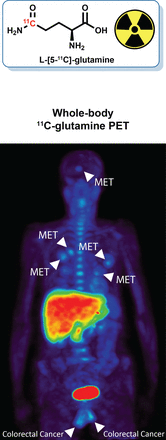
Visual Abstract
Abstract
Altered metabolism is a hallmark of cancer. In addition to glucose, glutamine is an important nutrient for cellular growth and proliferation. Noninvasive imaging via PET may help facilitate precision treatment of cancer through patient selection and monitoring of treatment response. l-[5-11C]-glutamine (11C-glutamine) is a PET tracer designed to study glutamine uptake and metabolism. The aim of this first-in-human study was to evaluate the radiologic safety and biodistribution of 11C-glutamine for oncologic PET imaging. Methods: Nine patients with confirmed metastatic colorectal cancer underwent PET/CT imaging. Patients received 337.97 ± 44.08 MBq of 11C-glutamine. Dynamic PET acquisitions that were centered over the abdomen or thorax were initiated simultaneously with intravenous tracer administration. After the dynamic acquisition, a whole-body PET/CT scan was acquired. Volume-of-interest analyses were performed to obtain estimates of organ-based absorbed doses of radiation. Results: 11C-glutamine was well tolerated in all patients, with no observed safety concerns. The organs with the highest radiation exposure included the bladder, pancreas, and liver. The estimated effective dose was 4.46E−03 ± 7.67E−04 mSv/MBq. Accumulation of 11C-glutamine was elevated and visualized in lung, brain, bone, and liver metastases, suggesting utility for cancer imaging. Conclusion: PET using 11C-glutamine appears safe for human use and allows noninvasive visualization of metastatic colon cancer lesions in multiple organs. Further studies are needed to elucidate its potential for other cancers and for monitoring response to treatment.
Altered metabolism has been shown to be important for cancer cell growth and proliferation (1,2). Conventional metabolic imaging has focused primarily on the role of glucose through PET imaging with 18F-FDG. However, studies have demonstrated the importance of additional metabolic pathways (2–8). This feature of cancer has led to the development of metabolism-targeted imaging and therapeutic strategies focused on pathways other than glycolysis (3–5,7–14). Noninvasive molecular imaging with novel PET tracers is increasingly being deployed in clinical oncology. Targeting tumor-specific pathways represents a promising approach for improved PET imaging of tumors.
Glutamine represents an important metabolic substrate that is dysregulated in cancer (3–9,11–14). Glutamine metabolism allows for energy production via adenosine triphosphate, anaplerosis through the tricarboxylic acid cycle, defense against oxidative stress via glutathione, and biosynthesis of other amino acids and nucleotides (3–9,12). In oncology, glutamine is transported into cells primarily by ASCT2, the sodium-dependent neutral amino acid transporter encoded by SLC1A5. ASCT2 is overexpressed in several cancer types (12–14), and this characteristic has been linked to poor survival (13). Imaging of glutamine could be complementary to 18F-FDG imaging by identifying tumors that either are 18F-FDG–negative or are in locations with high background 18F-FDG uptake (7). In addition, glutamine imaging could provide further information about the cancer’s underlying biology. Finally, it could serve as a tool for new therapies targeting glutamine metabolism (15,16).
Syntheses of both 18F- and 11C-labeled glutamine have been reported (17–27). 18F-glutamine has been studied preclinically (28–39) and clinically (31,36,40–43). However, the distribution and metabolism of 18F-glutamine differ from those of the naturally occurring substrate, and 18F-glutamine is prone to defluorination in vivo (28,31,40). l-[5-11C]-glutamine (11C-glutamine) is chemically and biologically identical to physiologic glutamine. Cells that avidly take up glutamine will also avidly take up 11C-glutamine, thereby providing a direct marker of glutamine transport and the first step of glutaminolysis. 11C-glutamine has been studied in preclinical mouse models (21) but to date has not been studied in humans. Here, we report the first-in-human studies using 11C-glutamine in a clinical trial of patients with colorectal cancer.
MATERIALS AND METHODS
Patients
Patients were prospectively enrolled in a clinical trial conducted at Vanderbilt University Medical Center (VUMC, ClinicalTrials.gov identifier NCT03263429) and underwent baseline (pretreatment) PET/CT imaging. The trial was approved by VUMC’s Institutional Review Board and all subjects provided written informed consent before participating in the study. The study was conducted in accordance with the Helsinki Declaration and the Health Insurance Portability and Accountability Act. All patients were at least 18 y old, had a histologically or cytologically confirmed diagnosis of metastatic wild-type KRAS colorectal cancer, and had received prior antiepidermal growth factor receptor therapy. In addition, patients had undergone baseline evaluation of disease status by CT or MRI and had at least 1 measurable lesion as defined by RECIST 1.1.
11C-Glutamine Production
11C-glutamine was synthesized by the VUMC Radiochemistry Core as previously described (27) under current good-manufacturing-practice conditions with approval from the VUMC Radioactive Drug Research Committee. 11C-glutamine met all U.S. Pharmacopeia chapter 823 requirements for a sterile, injectable PET radiopharmaceutical. Quality control included analysis of radiochemical and chemical purities, residual solvent content, endotoxin content, pH, filter integrity, radionuclidic purity, and appearance. Sterility testing was performed after release. The production method yields mass levels below 800 μg, which falls several orders of magnitude below reported safe dose levels (44–46). Molar activity was not measured given that these mass levels fall below the detection limit of the instrumentation.
Imaging Protocol
Images were acquired using a Philips Vereos PET/CT scanner with patients lying supine. A dynamic imaging protocol was conducted before a whole-body protocol. Patients were asked to fast for at least 6 h before tracer injection, and blood glucose levels were tested before administration of radioactivity. The PET acquisition was initiated simultaneously with intravenous injection of 11C-glutamine over 30 s. The mean administered activity (±SD) was 337.97 ± 44.08 MBq (range, 232.36–386.98 MBq). Dynamic emission images were acquired over the tumor region of interest using six 1-min scans, six 2-min scans, six 5-min scans, and one 10-min scan, for a total duration of 58 min, as tolerated by the patient. A whole-body (vertex of skull to mid thighs) PET scan was then acquired using 9 bed positions, at 2 min per bed position, for a total scan time of about 18 min. Before and accompanying each PET image exam, a brief low-energy, whole-body transmission CT scan without contrast medium (120 kVp, 25 mAs, and 4.0-mm slice thickness) was acquired for attenuation correction and anatomic localization. PET images were reconstructed using iterative ordered-subset expectation maximization (15 subsets, 3 iterations), with all corrections applied. The reconstructed PET images had a 4-mm slice thickness and a 169 × 169 transaxial matrix with 4-mm pixel spacing. Patients were monitored during and for 24 h after the PET scan for any reactions or adverse events. Side effects and reactions were graded per the Common Terminology Criteria for Adverse Events, version 4.03. There were no adverse or clinically detectable pharmacologic effects in any of the 9 subjects.
Image Analysis
Maximum-intensity-projection and PET/CT images were viewed using OsiriX (Pixmeo). Regions of interest were drawn over lesions, normal liver, and the left ventricular blood pool. The ratios of the maximum value in the lesion to the average value in the blood pool from whole-body images were documented for each lesion for the patients shown in the figures. The ratios of the maximum value in the lesion to the average value in the normal liver from whole-body images are given for the liver lesions shown in the figures. A volume-of-interest (VOI) analysis was performed for both the dynamic and the whole-body imaging protocols using the Inveon Research Workplace, version 4.2 (Siemens Medical Solutions USA). For analysis of the dynamic PET images, VOIs were drawn over the organs that were within the dynamic PET protocol’s field of view, which consisted of 1 bed position and spanned either the lungs or the abdomen. The mean activity concentration (Bq/mL) in each VOI was decay-corrected to the beginning of the study to generate time–activity curves over the duration of the scan. Those patients whose abdomen was within the PET field of view during the dynamic PET protocol were suitable for radiation dosimetry estimation. For the whole-body imaging protocol, VOIs were drawn over organs throughout the entire body. The mean concentration (Bq/mL) and SUV using the patient’s body weight were calculated for each VOI.
Patient-to-Phantom Data Conversion
To convert patient %ID/g ( ) values to phantom whole-organ %ID/organ (
) values to phantom whole-organ %ID/organ ( ) for use in estimating whole-organ cumulative activities, Equation 1 was used:
) for use in estimating whole-organ cumulative activities, Equation 1 was used: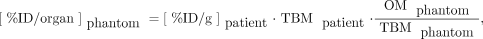 Eq. 1where
Eq. 1where 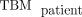 is the total-body mass of the patient,
is the total-body mass of the patient,  is the phantom organ mass (from the OLINDA organ mass listing (47,48) and Reference Man (49)), and
is the phantom organ mass (from the OLINDA organ mass listing (47,48) and Reference Man (49)), and  is the phantom total-body mass (adult male, 74 kg; adult female, 57 kg). This approach assumes that the concentration of activity in a tissue relative to the overall concentration in the whole body is preserved when translating from patient to phantom.
is the phantom total-body mass (adult male, 74 kg; adult female, 57 kg). This approach assumes that the concentration of activity in a tissue relative to the overall concentration in the whole body is preserved when translating from patient to phantom.
Cumulative Activity Calculation
Cumulative activity for each organ was calculated from the biokinetic curves of the dynamic PET scans by first multiplying each point by 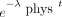 , where
, where  is the decay constant for 11C, 2.04 h−1, and t is the postinjection time of the data point. Piecewise numeric integration using the trapezoidal method was applied to calculate the cumulative activity for each organ. The cumulative activity from
is the decay constant for 11C, 2.04 h−1, and t is the postinjection time of the data point. Piecewise numeric integration using the trapezoidal method was applied to calculate the cumulative activity for each organ. The cumulative activity from  ,
,  , to the final time point,
, to the final time point, 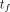 ,
, 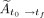 (Bq-h), was calculated by numerically integrating the tissue radioactivity using the trapezoidal rule in Equation 2:
(Bq-h), was calculated by numerically integrating the tissue radioactivity using the trapezoidal rule in Equation 2: Eq. 2
Eq. 2
The cumulative activity from the time of the final scan time point, 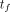 , to infinity,
, to infinity, 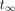 ,
,  (Bq-h), was calculated by dividing the activity after the final scan, A(tf), by the physical decay constant,
(Bq-h), was calculated by dividing the activity after the final scan, A(tf), by the physical decay constant,  :
: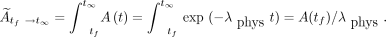 Eq. 3
Eq. 3
The total cumulative activity, 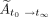 (Bq-h), was then calculated by summing
(Bq-h), was then calculated by summing 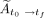 and
and 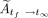 :
: Eq. 4
Eq. 4
The number of decays per injected activity (Bq-h/Bq) was computed for each organ by dividing the cumulative activity by the injected activity (Bq). Furthermore, the number of decays per injected activity for the remainder was computed by subtracting the sum of organ cumulative activities from the total number of decays from 1 Bq of 11C, assuming only physical decay. Eq. 5
Eq. 5
Absorbed Dose Estimation
Given the residence times, OLINDA 1.1 (48) was used to estimate the absorbed doses in adult phantoms. Absorbed dose per injected activity (mGy/MBq) was estimated for all organs of interest. The decays in the bladder were assumed to equal the decay-corrected whole-body fraction of injected activity in the bladder for the whole-body PET scan multiplied by τ, where τ = 1 Bq/λphys, 0.49 h
RESULTS
Safety and Biodistribution
Baseline 11C-glutamine imaging data from 9 patients were evaluated. There were no signs of toxicity or observed adverse events after injection of 11C-glutamine. Whole-body PET analysis in normal organs included evaluation of uptake in the salivary glands, heart, bladder, liver, spleen, kidneys, pancreas, stomach, small intestine, lungs, muscle, fat, brain, bone marrow, testis, and adrenals (Fig. 1; Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). The highest signal was observed in the bladder, as was expected because of excretion of the tracer. High activity was also seen in the pancreas and liver. Examination of the time–activity curves showed distinctive clearance profiles for different organs (Supplemental Fig. 2). High initial activity was seen in the kidneys (Supplemental Figs. 2A and 2B), spleen (Supplemental Fig. 2C), and heart (Supplemental Fig. 2D), with rapid clearance observed in all these organs. The liver showed a gradual increase in activity up to 5–15 min, followed by a slow washout (Supplemental Fig. 2E), whereas uptake in the pancreas increased up to 11–13 min and then plateaued (Supplemental Fig. 2F). There was low intestinal uptake, with activity peaking rapidly and then remaining stable up to the end of the dynamic scan (Supplemental Fig. 2G). Large variability was seen in the 11C-glutamine time–activity curves for bone marrow in these patients (Supplemental Fig. 2H). There was variable uptake in the adrenals for different patients, but all curves followed the same pattern (Supplemental Fig. 2I).
Whole-body biodistribution of 11C-glutamine uptake in normal tissues. Activity concentration (Bq/mL) is plotted for 1 h after injection.
Estimated Absorbed Dose
The average number of decays per injected activity (Bq-h/Bq) for each organ is provided in Table 1. Table 2 provides the average absorbed dose per injected activity (mGy/MBq) for adults. The mean effective dose for 11C-glutamine (±SD) was 4.46E−03 ± 7.67E− 04 mSv/MBq. The organs with the highest doses were the liver (1.68E−02 ± 4.69E−03 mGy/MBq), pancreas (1.30E−02 ± 2.64E−03 mGy/MBq), and bladder wall (1.14E−02 ± 3.45E−03 mGy/MBq). The only organ whose coefficient of variation was greater than 30% was the adrenal glands, at 35%.
Cumulative Activity of 11C-Glutamine in Organs of Interest for Each Patient
Dosimetry Estimates for Adults Based on Dynamic PET Imaging with 11C-Glutamine
Uptake in Metastatic Lesions
Accumulation of 11C-glutamine exceeded the background level in several lesions across subjects presenting with pulmonary metastases (Figs. 2A, 2B, 3C, and 3D; Supplemental Fig. 3D) and hepatic metastases (Fig. 4C). Visualization of normal-organ accumulation in the whole-body images of a representative patient illustrating high uptake in the bladder, liver, and pancreas is shown in Figures 3A and 3B. 11C-glutamine uptake in hepatic lesions was often at the periphery with a photopenic center, which existed on a high background of normal accumulation (Figs. 4A–4C). Some liver metastases and an adrenal mass were indistinguishable from background liver accumulation but could be imaged with an alternative tracer of glutamine metabolism (Supplemental Figs. 3A–3C and 4) (50,51). Skeletal metastases were also observed (Figs. 4A, 4B, and 4D). Interestingly, previously unidentified brain metastases were seen in 2 patients (Fig. 2C) and were subsequently confirmed using standard imaging (Fig. 2D). Glutamine-avid lesions typically exhibited rapid postinjection accumulation, which either plateaued or gradually decreased over time (Fig. 4E). Representative 11C-glutamine tumor uptake values (lesion–to–blood pool ratios) are given in Table 3.
11C-glutamine tumor uptake in patient with metastatic colorectal cancer. (A–C) Axial 11C-glutamine PET/CT images corresponding to left lung metastasis (A), 2 right lung metastases (B), and brain metastasis (C). Arrowheads point to lesions. Lesion-to-blood pool ratios from whole-body scan were 2.64 (A), 2.22 (B, top arrow), 2.35 (B, bottom arrow), and 1.68 (C). (D) Contrast-enhanced MRI 6.5 wk after baseline PET imaging and treatment. Lesion is indicated with arrowhead. MRI confirms presence of brain lesion seen with 11C-glutamine PET.
11C-glutamine biodistribution and tumor imaging in patient with metastatic colorectal cancer. (A and B) Whole-body PET (A) and PET/CT (B) images of 11C-glutamine showing normal-organ accumulation. High uptake was seen in bladder, liver, and pancreas. (C and D) Axial 11C-glutamine PET/CT fusion images corresponding to 2 lung nodules. Arrowheads point to lesions. Lesion–to–blood pool ratios from whole-body scan were 2.17 (C) and 2.59 (D).
11C-glutamine biodistribution and tumor imaging in patient with metastatic colorectal cancer. (A and B) Whole-body PET (A) and PET/CT (B) images of 11C-glutamine showing normal-organ accumulation and several metastases. High uptake was seen in bladder, liver, and pancreas. (C and D) Axial 11C-glutamine PET/CT fusion images corresponding to liver metastasis (C) and left humeral head metastasis (D). Arrowheads point to lesions. Lesion–to–blood pool ratios from whole-body scan were 5.33 (C) and 4.02 (D). Lesion-to-liver ratio from whole-body scan was 1.66 (C). Time–activity curves (E) for aorta, liver, liver lesions, and bone metastasis.
Quantification of 11C-Glutamine Uptake in Tumors
DISCUSSION
The role of altered metabolism in cancer progression is increasingly recognized (1,2). Altered glucose metabolism is a well-known phenomenon, but recently there has been growing emphasis on other pathways, including amino acid metabolism. As the most abundant amino acid in the plasma, glutamine has been shown to be vital to the growth and survival of certain cancers (3–9,11–14). Thus, glutaminolysis has emerged as a novel therapeutic target (3,4,7–9,11–14). However, methods to predict responders or monitor response to these new therapies are lacking. Novel imaging methods can be developed as biomarkers to meet this unmet need and aid in the pursuit of precision approaches to oncology. Previous studies have reported imaging with 11C-labeled glutamine in preclinical models (21). Here, we present the clinical translation of this agent.
As part of this first-in-human study, we evaluated the safety and biodistribution of 11C-glutamine in patients with metastatic colorectal cancer. 11C-glutamine was found to be safe, with no adverse side effects observed at the dose used in this study. Uptake of 11C-glutamine was highest in the bladder, liver, and pancreas. High uptake in the pancreas is consistent with previously reported biodistribution studies in mice (21). This uptake was attributed to the exocrine function and high protein turnover within the pancreas. Additionally, clearance profiles were comparable to those found in mice, with the heart and kidneys demonstrating rapid uptake and clearance but the liver showing a slower washout (21). Excretion through the bladder was also observed preclinically. The biodistribution pattern of 11C-glutamine is consistent with the human biodistribution of another 11C-labeled amino acid, l-[methyl-11C]methionine (52).
Use of 11C-glutamine has several potential advantages, including the opportunity to leverage the short half-life of 11C to follow other orthogonal metabolic pathways through simple sequential imaging protocols (51). Although certain technical advantages are inherent to the fluorinated agent, 18F-(2S,4R)-4-fluoroglutamine, this tracer and 11C-glutamine differ with respect to metabolic fate. For example, accumulated 18F-(2S,4R)-4-fluoroglutamine remains as the parent compound, with a small fraction resulting from metabolites (28,31,34,40,43). Only a percentage is incorporated into biomolecules (28,29,34,38,40). An aliphatic fluoride-labeled analog, 18F-(2S,4R)-4-fluoroglutamine is also prone to defluorination in vivo (28,31,37,38,40,43). In contrast, 11C-glutamine is metabolized to 11C-glutamate and 11C-CO2 (53), as well as incorporated directly into biomolecules (21). Generally, imaging with 11C-glutamine reports on both uptake and downstream metabolism, whereas uptake of 18F-(2S,4R)-4-fluoroglutamine is likely more representative of glutamine transport (34). Thus, while 11C-glutamine behaves identically to the naturally occurring substrate, this characteristic also represents a potential limitation of this tracer for PET imaging, as the signal detected will be from all radioactively labeled molecules, including the parent ligand and a range of metabolic intermediates.
The mean effective dose for 11C-glutamine (±SD) was 4.46E− 03 ± 7.67E−04 mSv/MBq. Although a full range of time points across the whole body were not collected in this study, our calculated value is comparable to other reported effective doses for 11C-labeled PET tracers (52,54–57). This effective dose is similar to those reported for other 11C-labeled amino acid radiopharmaceuticals (52,54,55) and a magnitude lower than dose estimates for 18F-labeled radiopharmaceuticals found in the literature (50,58–62), due mainly to the short half-life of 11C. This value compares well with the mean effective dose (5.9E−03 ± 2.0E−03 mSv/MBq) reported in a review of 37 dose estimates for 11C-labeled PET tracers (56) and with the average effective dose (5.2E−03 ± 1.7E−03 mSv/MBq) from a recent review of 77 literature publications for a wide range of 11C-labeled tracers (57). It is approximately one fourth the average effective dose (2.05E−02 ± 7.6E−03 mSv/MBq) found from a review of 144 publications for a range of 18F-labeled tracers (57). The effective dose estimate for 11C-glutamine is about 4-fold less than that for 18F-(2S,4R)-4-fluoroglutamine (42). Because the magnitudes of the SUVs of the 2 radiotracers are very similar (40), the difference in dosimetry can be attributed mainly to the difference in physical half-lives.
11C-glutamine enabled the visualization of tumor lesions in various metastatic sites, including the liver, lungs, bones, and brain. Consistent with these data, prior human studies with 18F-(2S,4R)-4-fluoroglutamine also showed tracer uptake in brain, bone, and lung metastases (40,41,43). Uptake of 18F-(2S,4R)-4-fluoroglutamine in normal liver was more intense than that in liver lesions, with the tumor sites appearing as cold spots (41). This pattern matches the uptake pattern we observed for many of the liver lesions in our study using 11C-glutamine. In hepatic metastases, normal-liver uptake can exceed tumor uptake, making it difficult to define tumor lesions. This characteristic represents a potential limitation of these tracers. A potential advantage of 11C-glutamine could be in the detection of bone metastases, as these lesions could be missed with 18F-(2S,4R)-4-fluoroglutamine because of normal uptake of free 18F in the bone marrow through defluorination of the tracer (41). 18F-(2S,4R)-4-fluoroglutamine enabled visualization of breast cancer lymph node metastases as well (36,42). The utility of 11C-glutamine in other cancer types was not evaluated in this work and will be the focus of future studies. Not all lesions identified in these patients were glutamine-avid. Thus, 11C-glutamine PET may inform each metastatic tumor’s underlying metabolism and biology. This intrapatient tumoral heterogeneity emphasizes the need for a noninvasive means of diagnosis and staging and points to the importance of using multiple orthogonal approaches to studying cancer. Imaging with 11C-glutamine can complement other imaging approaches, including 18F-FDG PET and PET imaging with other tracers currently in development, thus providing a more complete picture of the patient’s disease and underlying biologic processes at individual metastatic sites. The use of total-body PET scanners could also potentially result in images with higher sensitivity for lesion detection and improved estimation of kinetic parameters by enabling simultaneous dynamic imaging of multiple organs or lesions (63–65). However, these scanners are still under development and not yet widely available.
One limitation of this study was the small sample size and evaluation within a focused clinical context (metastatic colorectal cancer). In addition, full quantitative analyses of tumor uptake were not performed. Thus, the diagnostic utility of 11C-glutamine PET in colorectal cancer remains to be determined and is an area for future investigation. It is likely that other solid tumors may be effectively imaged with this tracer, which may also provide insight into tumors that may be sensitive to certain metabolically targeted therapies such as inhibitors of glutaminase activity (16) or glutamine transport (15). The utility of 11C-glutamine PET in imaging tumors beyond colorectal cancer and in monitoring treatment response should be the focus of future work.
CONCLUSION
This clinical study demonstrated that 11C-glutamine is well tolerated in humans. PET imaging with 11C-glutamine is feasible, and elevated uptake was seen in lesions of patients with metastatic colorectal cancer. The estimated dosimetry is consistent with that of other 11C-labeled tracers. Thus, further use of this tracer is warranted. Larger clinical studies will provide additional information and could demonstrate the utility of 11C-glutamine for imaging in a variety of cancer types and for measurement of treatment response.
DISCLOSURE
This work was supported by grants from the NIH (P30 CA068485, P50 CA236733, U24 CA220325, R35 CA197570, S10 OD019963, and S10 OD012297) and generous philanthropic funding from the Julia Reed Foundation, Debbie and Michael Rose Discovery Grant, and Samuel K. and Sandra G. Wellborn Cancer Discovery Grant. H. Charles Manning is a Cancer Prevention Research Institute of Texas (CPRIT) Scholar in Cancer Research and is supported by CPRIT RR200046. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 11C-glutamine safe and suitable for PET imaging of human cancer?
PERTINENT FINDINGS: In this study evaluating the safety and biodistribution of 11C-glutamine and analyzing its ability to visualize metastatic lesions in patients with colorectal cancer, 11C-glutamine was well tolerated and showed increased uptake in tumors relative to background.
IMPLICATIONS FOR PATIENT CARE: Imaging with 11C-glutamine may advance precision medicine by enabling the characterization of tumors noninvasively by PET and may serve as a predictive and prognostic biomarker in future studies.
ACKNOWLEDGMENTS
We thank the patients and their families for participating in this study. Anna Fisher and Adam Rosenberg are acknowledged for experimental and manuscript support. The VUMC/VUIIS Human Imaging and Radiochemistry Core facilities assisted with PET and preparation of the radiotracer.
Footnotes
Published online April 30, 2021.
- © 2022 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 23, 2020.
- Revision received March 26, 2021.