Visual Abstract
Abstract
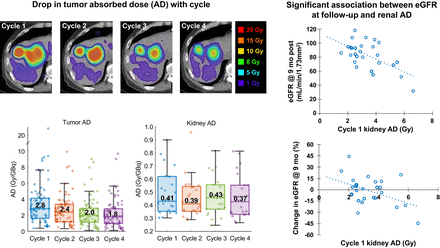
We investigated pharmacokinetics, dosimetric patterns, and absorbed dose (AD)–effect correlations in [177Lu]Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) for metastatic neuroendocrine tumors (NETs) to develop strategies for future personalized dosimetry-guided treatments. Methods: Patients treated with standard [177Lu]Lu-DOTATATE PRRT were recruited for serial SPECT/CT imaging. Kidneys were segmented on CT using a deep learning algorithm, and tumors were segmented at each cycle using a SPECT gradient-based tool, guided by radiologist-defined contours on baseline CT/MRI. Dosimetry was performed using an automated workflow that included contour intensity–based SPECT-SPECT registration, generation of Monte Carlo dose-rate maps, and dose-rate fitting. Lesion-level response at first follow-up was evaluated using both radiologic (RECIST and modified RECIST) and [68Ga]Ga-DOTATATE PET-based criteria. Kidney toxicity was evaluated based on the estimated glomerular filtration rate (eGFR) at 9 mo after PRRT. Results: Dosimetry was performed after cycle 1 in 30 patients and after all cycles in 22 of 30 patients who completed SPECT/CT imaging after each cycle. Median cumulative tumor (n = 78) AD was 2.2 Gy/GBq (range, 0.1–20.8 Gy/GBq), whereas median kidney AD was 0.44 Gy/GBq (range, 0.25–0.96 Gy/GBq). The tumor-to-kidney AD ratio decreased with each cycle (median, 6.4, 5.7, 4.7, and 3.9 for cycles 1–4) because of a decrease in tumor AD, while kidney AD remained relatively constant. Higher-grade (grade 2) and pancreatic NETs showed a significantly larger drop in AD with each cycle, as well as significantly lower AD and effective half-life (Teff), than did low-grade (grade 1) and small intestinal NETs, respectively. Teff remained relatively constant with each cycle for both tumors and kidneys. Kidney Teff and AD were significantly higher in patients with low eGFR than in those with high eGFR. Tumor AD was not significantly associated with response measures. There was no nephrotoxicity higher than grade 2; however, a significant negative association was found in univariate analyses between eGFR at 9 mo and AD to the kidney, which improved in a multivariable model that also adjusted for baseline eGFR (cycle 1 AD, P = 0.020, adjusted R2 = 0.57; cumulative AD, P = 0.049, adjusted R2 = 0.65). The association between percentage change in eGFR and AD to the kidney was also significant in univariate analysis and after adjusting for baseline eGFR (cycle 1 AD, P = 0.006, adjusted R2 = 0.21; cumulative AD, P = 0.019, adjusted R2 = 0.21). Conclusion: The dosimetric behavior we report over different cycles and for different NET subgroups can be considered when optimizing PRRT to individual patients. The models we present for the relationship between eGFR and AD have potential for clinical use in predicting renal function early in the treatment course. Furthermore, reported pharmacokinetics for patient subgroups allow more appropriate selection of population parameters to be used in protocols with fewer imaging time points that facilitate more widespread adoption of dosimetry.
Footnotes
Published online Apr. 24, 2025.
- © 2025 by the Society of Nuclear Medicine and Molecular Imaging.
This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
SNMMI members
Login to the site using your SNMMI member credentials
Individuals
Login as an individual user